75091
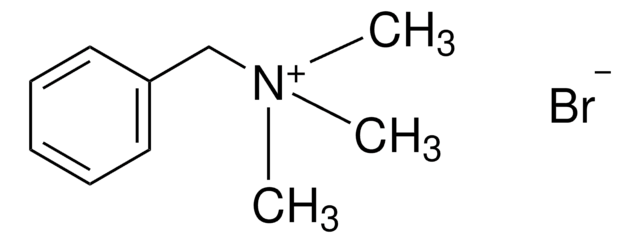
Trimethyloctylammonium bromide
≥98.0% (AT)
Synonym(s):
Octyltrimethylammonium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)7N(CH3)3(Br)
CAS Number:
Molecular Weight:
252.23
Beilstein:
3628448
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (AT)
form
powder
SMILES string
[Br-].CCCCCCCC[N+](C)(C)C
InChI
1S/C11H26N.BrH/c1-5-6-7-8-9-10-11-12(2,3)4;/h5-11H2,1-4H3;1H/q+1;/p-1
InChI key
XCOHAFVJQZPUKF-UHFFFAOYSA-M
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sören Großkopf et al.
Langmuir : the ACS journal of surfaces and colloids, 35(8), 3048-3057 (2019-02-01)
In the present work, we study the shear-induced transformation of polymer-rich lamellar phases into vesicles. The evolution of vesicle size is studied by different scattering techniques, rheology, and microscopy methods. The lamellar phase found in the system D2O/ o-xylene/ Pluronic
David Zanuy et al.
Biopolymers, 63(3), 151-162 (2002-01-12)
We present a molecular dynamics simulation at 300 K in explicit solvent environment of chloroform of the stoichiometric complex formed by poly(alpha,L-glutamate) and octyltrimethylammonium ions. We observed that the alpha-helix conformation of the polypeptide chain remains stable during a 2-ns
Yueh-Feng Li et al.
Chemosphere, 266, 128949-128949 (2020-12-08)
Perfluorooctanoic acid (PFOA) was separated and recovered using a foam flotation process aided by cationic surfactants. The PFOA removal efficiency was in the following decreasing order: OTAB (C8TAB) > DTAB (C10TAB) > CTAB (C16TAB) > TBAB, which indicates that cationic surfactants with an alkyl chain that
Sudhir Husale et al.
Nucleic acids research, 36(5), 1443-1449 (2008-01-22)
The interaction of cationic surfactants with single dsDNA molecules has been studied using force-measuring optical tweezers. For hydrophobic chains of length 12 and greater, pulling experiments show characteristic features (e.g. hysteresis between the pulling and relaxation curves, force-plateau along the
N B Cech et al.
Analytical chemistry, 73(19), 4632-4639 (2001-10-19)
The effect of uneven fissioning of mass and charge from electrospray droplets on the amount of analyte charged during the electrospray process was explored. A surface selectivity factor (S) was developed to describe the affinity of an analyte for the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service