762016
3-Azido-1-propanamine
≥95%
Synonym(s):
3-Azidopropylamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H8N4
CAS Number:
Molecular Weight:
100.12
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.471
density
1.020 g/mL at 25 °C
storage temp.
−20°C
SMILES string
NCCCN=[N+]=[N-]
InChI
1S/C3H8N4/c4-2-1-3-6-7-5/h1-4H2
InChI key
OYBOVXXFJYJYPC-UHFFFAOYSA-N
General description
3-Azido-1-propanamine can be used to functionalize:
- Bismethylolpropionic acid (bis-MPA) monomers with azide functional group to generate high-generation dendrimers.,
- Clickable zinc tetraphenylporphyrin scaffold with an azido group through click chemistry applicable in photodynamic therapy.
Application
Amine modified azide for click chemistry.
3-Azido-1-propanamine may be used in the synthesis of mannopyranoside dendrimers for studying multivalent carbohydrate-protein interactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
140.0 °F
Flash Point(C)
60 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of mono-, di-and triporphyrin building blocks by click chemistry for photodynamic therapy application
Gazzali AM, et al.
Tetrahedron, 73(5), 532-541 (2017)
Two-dimensional ultrafast vibrational spectroscopy of azides in ionic liquids reveals solute-specific solvation
Dutta S, et al.
Physical Chemistry Chemical Physics, 17(40), 26575-26579 (2015)
Dual display of proteins on the yeast cell surface simplifies quantification of binding interactions and enzymatic bioconjugation reactions.
Lim S, et al.
Biotechnology Journal, 12(5) (2017)
Synthesis and solvodynamic diameter measurements of closely related mannodendrimers for the study of multivalent carbohydrate?protein interactions.
Chabre YM, et al.
Beilstein Journal of Organic Chemistry, 10, 1524-1524 (2014)
Mariano Ortega-Muñoz et al.
Nanoscale, 11(16), 7850-7856 (2019-04-10)
Activated carbon nanodots functionalized with acid anhydride groups (AA-CNDs) are prepared by one-pot water-free green thermolysis of citric acid. As a proof of concept of their capabilities as appealing and versatile platforms for accessing engineering nanoconstructs, the as-prepared AA-CNDs have
Articles
Explore the principles and applications of click chemistry in drug discovery, highlighting efficient reactions that streamline the synthesis of bioactive compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service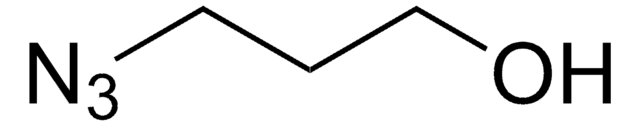


![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)