803863
Schreiner′s Thiourea Catalyst
95%
Synonym(s):
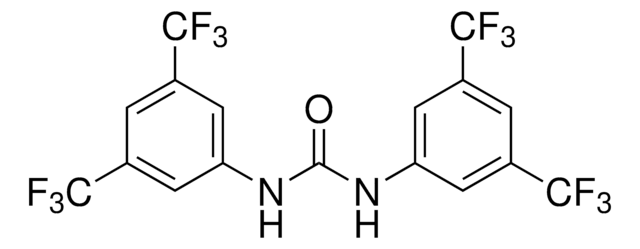
N,N′−bis[3,5−bis(trifluoromethyl)phenyl]−Thiourea
About This Item
Recommended Products
Quality Level
Assay
95%
form
powder or crystals
reaction suitability
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
170 °C
greener alternative category
SMILES string
S=C(NC1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1)NC2=CC(C(F)(F)F)=CC(C(F)(F)F)=C2
InChI
1S/C17H8F12N2S/c18-14(19,20)7-1-8(15(21,22)23)4-11(3-7)30-13(32)31-12-5-9(16(24,25)26)2-10(6-12)17(27,28)29/h1-6H,(H2,30,31,32)
InChI key
RWXWQJYJWJNJNW-UHFFFAOYSA-N
Related Categories
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Boronate ureas benefit from internal Lewis acid coordination of the urea cabonyl oxygen and the strategically placed boron. As a result of this structural feature, boronate ureas can be rendered more acidic than conventional urea hydrogen bond donor catalysts.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1,3-Bis[3,5-bis(trifluoromethyl)phenyl]thiourea](/deepweb/assets/sigmaaldrich/product/structures/191/427/0218c99c-65b9-4963-938c-c47a5790dfc5/640/0218c99c-65b9-4963-938c-c47a5790dfc5.png)
![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(−)-2-(dimethylamino)cyclohexyl]thiourea](/deepweb/assets/sigmaaldrich/product/structures/384/772/d336462c-f438-446d-be0c-4064705213cc/640/d336462c-f438-446d-be0c-4064705213cc.png)

![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N,3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)
![N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-6′-methoxy-9-cinchonanyl]thiourea 90%](/deepweb/assets/sigmaaldrich/product/structures/634/236/e688c89f-a93b-4698-a6fc-48e479a875cb/640/e688c89f-a93b-4698-a6fc-48e479a875cb.png)
![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)