914460
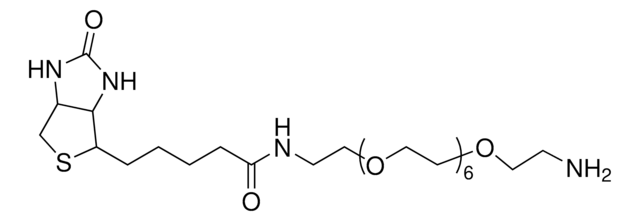
1-Biotinyl-3,6-dioxa-8-octaneamine hydrochloride
≥95%
Synonym(s):
1-Biotinyl-3,6-dioxa-8-octaneamine hydrochloride, N-(2-(2-(2-aminoethoxy)ethoxy)ethyl)-5-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide hydrochloride, N-{2-[2-(2- Aminoethoxy)ethoxy]ethoxy}biotinamide hydrochloride, Amine-terminated biotin linker, Biotin-DOOA HCl, Biotinylation reagent
About This Item
Recommended Products
Application
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Caged Cyclopropenes with Improved Tetrazine Ligation Kinetics
A Unified Framework for the Incorporation of Bioorthogonal Compound Exposure Probes within Biological Compartments
A Chemical Probe for Protein Crotonylation
One-Step Selective Exoenzymatic Labeling (SEEL) Strategy for the Biotinylation and Identification of Glycoproteins of Living Cells
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![O-(2-Aminoethyl)-O′-[2-(biotinylamino)ethyl]octaethylene glycol ≥95% (oligomer uniformity)](/deepweb/assets/sigmaaldrich/product/structures/300/798/e23e92b4-9ae0-4a8d-a37e-d57a8195a30e/640/e23e92b4-9ae0-4a8d-a37e-d57a8195a30e.png)