A56655
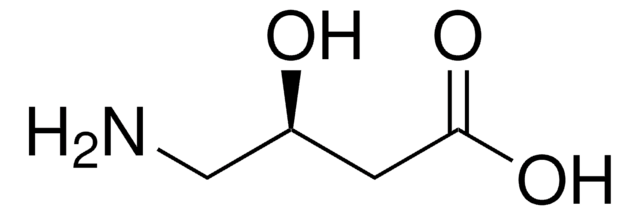
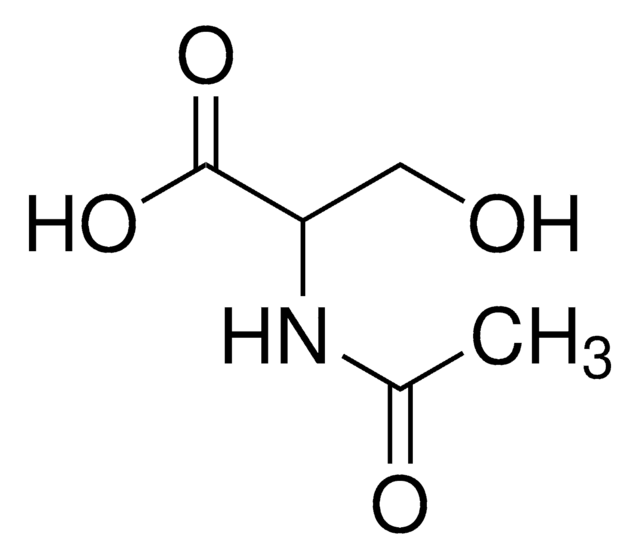
4-Amino-3-hydroxybutyric acid
98%
Synonym(s):
DL-γ-Amino-β-hydroxybutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CH(OH)CH2CO2H
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder or crystals
reaction suitability
reaction type: solution phase peptide synthesis
color
white to yellow
mp
223 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NCC(O)CC(O)=O
InChI
1S/C4H9NO3/c5-2-3(6)1-4(7)8/h3,6H,1-2,5H2,(H,7,8)
InChI key
YQGDEPYYFWUPGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Nakao et al.
Pharmacology, biochemistry, and behavior, 40(2), 359-366 (1991-10-01)
To investigate the formation of gamma-amino-beta-hydroxybutyric acid from 2-hydroxyputrescine in mammalian organs, the radioactive diamine was synthesized and was injected into rats intraperitoneally or intraventricularly. After intraperitoneal injection, the radioactive amino acid was detected in various organs, but formation of
G Bonardi et al.
Arzneimittel-Forschung, 31(11), 1910-1913 (1981-01-01)
1. Serum levels of DL-gamma-amino-beta-hydroxybutyric acid-1-14C (DL-GABOB-1-14C) in the rat (50 mg/kg) were quite similar after single i.v. and p.o. doses. Also the disappearance from the serum was similar with both administration routes. Within 6 days after p.o. treatment with
Elzbieta J Tadeusiak
Bioorganic chemistry, 32(6), 473-482 (2004-11-09)
The involvement of carnitine and gamma-amino-beta-hydroxybutyric acid in the biology of mammalian cells, the physiology of the human body, and some important aspects of medicinal treatment has induced many research groups to develop their pharmacologically potent analogues. Among them are
Stereoselective analysis of racemic psychotropic compounds by HPLC on chiral stationary phase.
D F Smith et al.
Psychopharmacology, 89(3), 392-393 (1986-01-01)
J Takahara et al.
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 12(1), 31-34 (1980-01-01)
Healthy male volunteers injected subcutaneously with 200 mg L-GABOB showed no significant changes in plasma GH, prolactin and cortisol levels. On the other hand, an intrathecal injection of 300 mg D, L-GABOB to cerebrovascular patients caused significant increases in plasma
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service