C102504
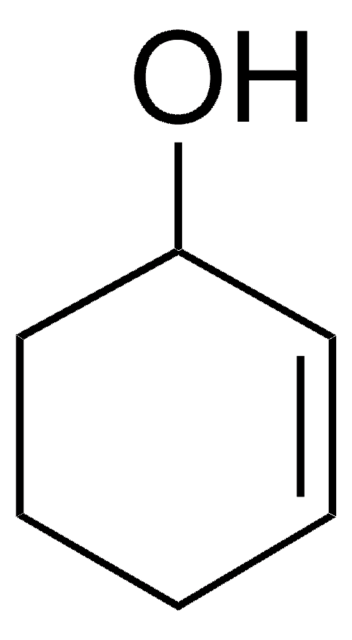
Cyclohexene oxide
98%
Synonym(s):
1,2-Epoxycyclohexane, 7-Oxabicyclo[4.1.0]heptane
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H10O
CAS Number:
Molecular Weight:
98.14
Beilstein:
383568
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
liquid
autoignition temp.
703 °F
expl. lim.
12.36 %
refractive index
n20/D 1.452 (lit.)
bp
129-130 °C (lit.)
density
0.97 g/mL at 25 °C (lit.)
SMILES string
C1CCC2OC2C1
InChI
1S/C6H10O/c1-2-4-6-5(3-1)7-6/h5-6H,1-4H2
InChI key
ZWAJLVLEBYIOTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Polymeric carbon nitride with internal np homojunctions for efficient photocatalytic CO2 reduction coupled with cyclohexene oxidation: This study focuses on the use of cyclohexene oxide in the context of photocatalytic CO2 reduction, highlighting the application of polymeric carbon nitride as a catalyst. The process shows how cyclohexene oxide can be efficiently converted in a coupled reaction that also addresses environmental concerns through CO2 reduction (W Zhen et al., 2021).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Muta. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liping Guo et al.
Dalton transactions (Cambridge, England : 2003), (27)(27), 5406-5410 (2009-07-01)
A new, natural lysine-based (salen)Cr(III)Cl ((lys-salen)Cr(III)Cl) complex was prepared and its catalytic activity for the copolymerization of CO(2) and cyclohexene oxide (CHO) was described in the presence of PPNCl (PPN(+) = bis(triphenylphosphoranylidene)ammonium) as cocatalyst. The influence of the reaction time
Matteo Proverbio et al.
Polymers, 11(7) (2019-07-25)
Cyclohexene oxide (CHO) and phthalic anhydride (PA) have been reacted in the presence of commercial salen-type complexes with different metals Cr (1), Al (2), and Mn (3) in combination with 4-(dimethylamino) pyridine (DMAP), bis-(triphenylphosphorydine) ammonium chloride (PPNCl) and bis-(triphenylphosphoranylidene)ammonium azide
Yoshiyuki Igawa et al.
Toxicology and applied pharmacology, 234(3), 361-369 (2008-11-26)
The polycyclic aromatic hydrocarbon 7, 12-dimethylbenz[a]anthracene, (DMBA), targets and destroys all follicle types in rat and mouse ovaries. DMBA requires bioactivation to DMBA-3,4-diol-1,2-epoxide for ovotoxicity via formation of the intermediate, DMBA-3,4-diol (catalyzed by microsomal epoxide hydrolase; mEH). mEH was shown
Benedetta Mennucci et al.
The Journal of organic chemistry, 72(18), 6680-6691 (2007-08-09)
The optical rotatory power of some natural cyclohexene oxides, such as (+)-chaloxone, 1, (+)-epiepoformine, 2, (+)-epoformine, 3, (+)-epoxidone, 5, (-)-sphaeropsidone, 6, (-)-episphaeropsidone, 7, and the synthetic compound (+)-epitheobroxide, 4, has been calculated by means of the TDDFT/B3LYP method using the
Kathila S Rajapaksa et al.
Toxicological sciences : an official journal of the Society of Toxicology, 96(2), 327-334 (2007-01-06)
Ovarian follicle disruption in mice caused by 7,12-dimethylbenz[a]anthracene (DMBA) is attributed to its bioactivation by CYP1B1 to a 3,4-epoxide which is then hydrolyzed to form a 3,4-diol by microsomal epoxide hydrolase (mEH). Further epoxidation by CYP1A1 or 1B1 forms the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![7-Oxabicyclo[4.1.0]heptan-2-one 98%](/deepweb/assets/sigmaaldrich/product/structures/209/639/448778d7-ca19-409d-a52e-8d2866c49812/640/448778d7-ca19-409d-a52e-8d2866c49812.png)







![7-Oxabicyclo[2.2.1]heptane 98%](/deepweb/assets/sigmaaldrich/product/structures/377/935/931d29d9-08c9-492a-b42e-3f8f5a20f595/640/931d29d9-08c9-492a-b42e-3f8f5a20f595.png)