D27004
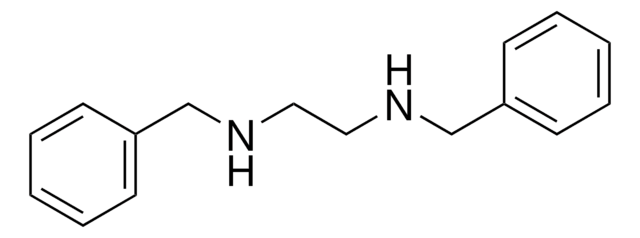
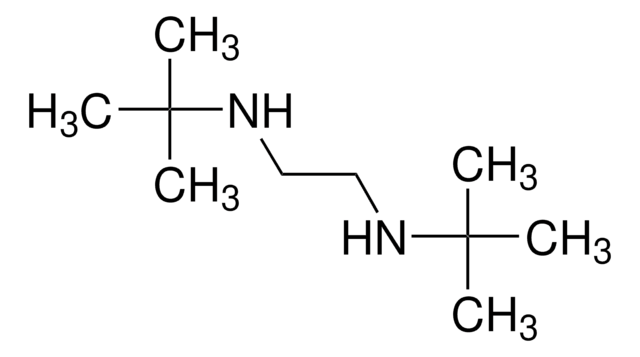
N,N′-Diphenylethylenediamine
98%
Synonym(s):
1,2-Dianilinoethane, N,N′-Ethylenedianiline, Wanzlick’s Reagent for aldehydes
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5NHCH2CH2NHC6H5
CAS Number:
Molecular Weight:
212.29
Beilstein:
646740
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
65-67 °C (lit.)
SMILES string
C(CNc1ccccc1)Nc2ccccc2
InChI
1S/C14H16N2/c1-3-7-13(8-4-1)15-11-12-16-14-9-5-2-6-10-14/h1-10,15-16H,11-12H2
InChI key
NOUUUQMKVOUUNR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N,N′-Diphenylethylenediamine can be used:
- To prepare nickel(II) chelates to study their chemical reactivities.
- To prepare N-heterocyclic carbene (NHC) adducts by reacting with substituted benzaldehydes.
- As a starting material to prepare substituted cyclic poly(methyl methacrylate)s.
Other Notes
Remainder mainly 1,4-diphenylpiperazine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yoshitane Imai et al.
Chemical communications (Cambridge, England), (10)(10), 1070-1072 (2006-03-04)
By using (1R,2R)-1,2-diphenylethylenediamine as a single enantiopure compound, we achieved a novel successive optical resolution of more than one kind of racemic compound through supramolecular crystallization.
P J Bednarski et al.
Drug metabolism and disposition: the biological fate of chemicals, 22(3), 419-427 (1994-05-01)
The cisplatin analog [meso-1,2-bis(2,6-dichloro-4-hydroxyphenyl) ethylenediamine]dichloroplatinum(II) [PtCl2(1)], by virtue of its estrogenic 1,2-diphenylethylenediamine ligand 1, was intended to function as a cytotoxic estrogen. This article reports on the reversible and irreversible interactions of this compound with plasma and plasma proteins in
Irina L Tourkova et al.
Laboratory investigation; a journal of technical methods and pathology, 97(9), 1072-1083 (2017-07-25)
To improve definition of the physical and hormonal support of bone formation, we studied differentiation of human osteoblasts in vitro at varying combinations of ACTH, 1α,25-dihydroxyvitamin D
Synthesis of cyclic poly (methyl methacrylate) by the intramolecular cyclization of α-amino, ω-carboxyl heterodifunctional poly (methyl methacrylate)
Kubo M, et al.
Polymer Bull., 47(1), 25-30 (2001)
Chemische Berichte, 86, 1463-1463 (1953)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,2,4]Triazolo[1,5-a][1,3,5]triazin-7-amine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/362/413/8a902135-3f29-47f0-8393-a194caf2c230/640/8a902135-3f29-47f0-8393-a194caf2c230.png)