G3407
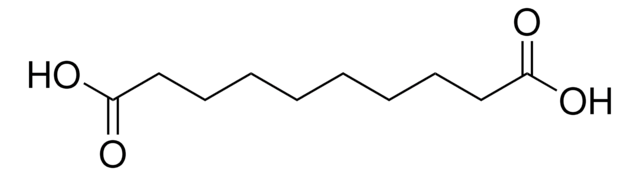
Glutaric acid
99%
Synonym(s):
1,3-Propanedicarboxylic acid, 1,5-Pentanedioic acid
About This Item
Recommended Products
Assay
99%
bp
200 °C/20 mmHg (lit.)
mp
95-98 °C (lit.)
solubility
water: soluble 5 mg/mL, clear to slightly hazy, colorless to faintly yellow
alcohol: soluble(lit.)
chloroform: soluble(lit.)
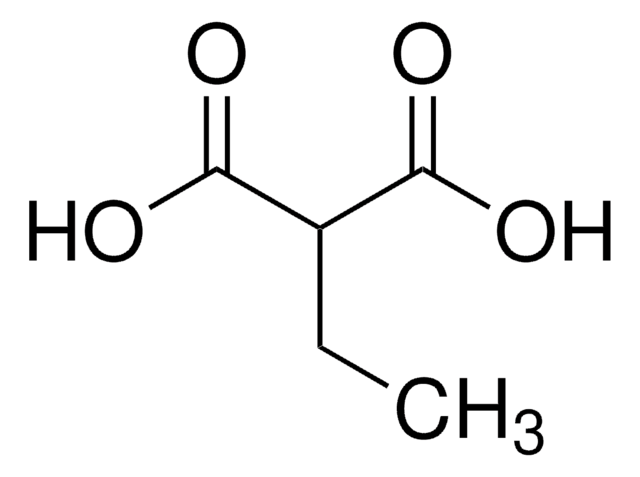
SMILES string
OC(=O)CCCC(O)=O
InChI
1S/C5H8O4/c6-4(7)2-1-3-5(8)9/h1-3H2,(H,6,7)(H,8,9)
InChI key
JFCQEDHGNNZCLN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Glutaric acid (Pentanedioic Acid) is a linear dicarboxylic acid. It has been prepared by oxidizing cyclopentane, cyclopentanol and cyclopentanone.
Application
- Complexation with DL-lysine. Complexes have been reported to possess zwitterionic lysinium ions (positively charged) and semi-glutarate ions (negatively charged).
- Synthesis of complexes with L-arginine and L-histidine.
- Preparation of glycine-glutaric acid co-crystals. Phase transition studies of these cocrystals have been reported by single-crystal X-ray diffraction, polarized Raman spectroscopy and differential scanning calorimetry.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service