H1529
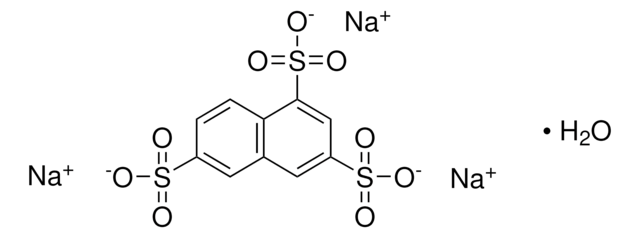
8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt
≥96%
Synonym(s):
HPTS, Pyranine, Solvent Green 7, Trisodium 8-hydroxypyrene-1,3,6-trisulfonate
About This Item
Recommended Products
description
Handling storage condition: Sightly light sensitive
Quality Level
Assay
≥96%
mp
>300 °C (lit.)
fluorescence
λex 454 nm; λem 511 nm (pH 9.1)
λex 454 nm; λem 520 nm in H2O
SMILES string
[Na+].[Na+].[Na+].Oc1cc(c2ccc3c(cc(c4ccc1c2c34)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O
InChI
1S/C16H10O10S3.3Na/c17-11-5-12(27(18,19)20)8-3-4-10-14(29(24,25)26)6-13(28(21,22)23)9-2-1-7(11)15(8)16(9)10;;;/h1-6,17H,(H,18,19,20)(H,21,22,23)(H,24,25,26);;;/q;3*+1/p-3
InChI key
KXXXUIKPSVVSAW-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Extruded fluorescent plastic indicator film for the detection of gaseous and dissolved CO2.
- Highly fluorescent waterborne polyurethane (WPU) matrix which is used as a pH sensing indicator.
- Positive (p-type) semiconducting polymeric gels via free radical cross-linking co-polymerization.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
481.3 °F
Flash Point(C)
249.6 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service