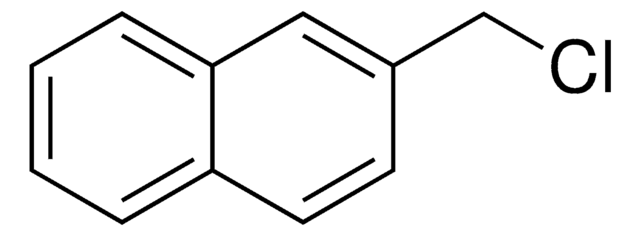
M57006
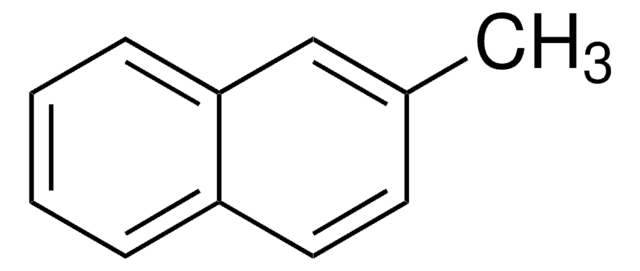
2-Methylnaphthalene (β)
95%
Synonym(s):
β-Methylnaphthalene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C10H7CH3
CAS Number:
Molecular Weight:
142.20
Beilstein:
906859
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
241-242 °C (lit.)
mp
34-36 °C (lit.)
density
1 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc2ccccc2c1
InChI
1S/C11H10/c1-9-6-7-10-4-2-3-5-11(10)8-9/h2-8H,1H3
InChI key
QIMMUPPBPVKWKM-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544) , CYP2A6(1548)
mouse ... Cyp2a5(13087)
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kazutoshi Shindo et al.
Bioscience, biotechnology, and biochemistry, 75(3), 505-510 (2011-03-11)
We performed combinational bioconversion of substituted naphthalenes with PhnA1A2A3A4 (an aromatic dihydroxylating dioxygenase from marine bacterium Cycloclasticus sp. strain A5) and prenyltransferase NphB (geranyltransferase from Streptomyces sp. strain CL190) or SCO7190 (dimethylallyltransferase from Streptomyces coelicolor A3(2)) to produce prenyl naphthalen-ols.
Franz D Bergmann et al.
Archives of microbiology, 193(4), 241-250 (2011-01-12)
The sulfate-reducing highly enriched culture N47 is capable to anaerobically degrade naphthalene, 2-methylnaphthalene, and 2-naphthoic acid. A proteogenomic investigation was performed to elucidate the initial activation reaction of anaerobic naphthalene degradation. This lead to the identification of an alpha-subunit of
Rainer U Meckenstock et al.
FEMS microbiology ecology, 49(1), 27-36 (2004-07-01)
Polycyclic aromatic hydrocarbons (PAHs) are among the most important contaminants of groundwater. The 2- and 3-ring PAHs are of particular concern because they are water soluble in the 1-200 mug/l range and are transported with the groundwater over significant distances.
Radosław Swiercz et al.
International journal of occupational medicine and environmental health, 23(4), 385-389 (2011-02-11)
The aim of the study was to evaluate the toxicokinetics of 2-methylnaphtalene (2-MN) during and after inhalation exposure. Male Wistar rats were exposed to 2-MN vapours at nominal concentrations of 200 or 400 mg/m3 in the dynamic inhalation chamber for
Drazenka Selesi et al.
Journal of bacteriology, 192(1), 295-306 (2009-10-27)
The highly enriched deltaproteobacterial culture N47 anaerobically oxidizes the polycyclic aromatic hydrocarbons naphthalene and 2-methylnaphthalene, with sulfate as the electron acceptor. Combined genome sequencing and liquid chromatography-tandem mass spectrometry-based shotgun proteome analyses were performed to identify genes and proteins involved
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service