All Photos(3)
About This Item
Empirical Formula (Hill Notation):
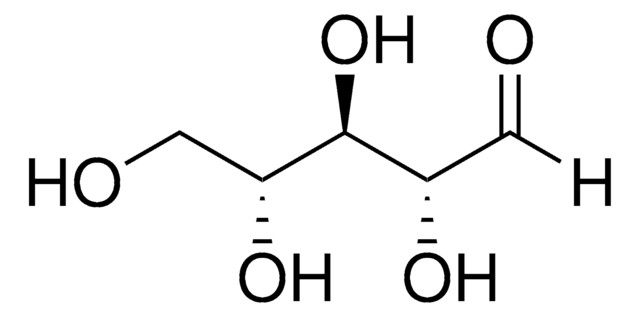
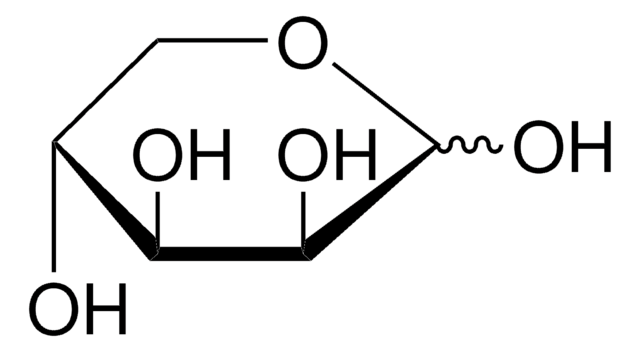
C5H10O5
CAS Number:
Molecular Weight:
150.13
Beilstein:
1723081
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
optical activity
[α]20/D −19.7°, c = 4 in H2O
mp
88-92 °C (lit.)
SMILES string
OC[C@@H](O)[C@@H](O)[C@@H](O)C([H])=O
InChI
1S/C5H10O5/c6-1-3(8)5(10)4(9)2-7/h1,3-5,7-10H,2H2/t3-,4+,5-/m0/s1
InChI key
PYMYPHUHKUWMLA-LMVFSUKVSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Raman K Sharma et al.
Bioorganic & medicinal chemistry, 20(23), 6821-6830 (2012-10-27)
A series of peracetylated O-aryl α,β-d-ribofuranosides have been synthesized and an efficient biocatalytic methodology has been developed for the separation of their anomers which was otherwise almost impossible by column chromatographic or other techniques. The incubation of 2,3,5-tri-O-acetyl-1-O-aryl-α,β-d-ribofuranoside with Lipozyme®
Thomas L Willett et al.
Bone, 52(2), 611-622 (2012-11-28)
Non-enzymatic glycation (NEG) and advanced glycation endproducts (AGEs) may contribute to bone fragility in various diseases, ageing, and other conditions by modifying bone collagen and causing degraded mechanical properties. In this study, we sought to further understand how collagen modification
Paul O Hassa et al.
Frontiers in bioscience : a journal and virtual library, 13, 3046-3082 (2007-11-06)
Poly-ADP-ribose metabolism plays a mayor role in a wide range of biological processes, such as maintenance of genomic stability, transcriptional regulation, energy metabolism and cell death. Poly-ADP-ribose polymerases (PARPs) are an ancient family of enzymes, as evidenced by the poly-ADP-ribosylating
Luigi J Alvarado et al.
Chembiochem : a European journal of chemical biology, 15(11), 1573-1577 (2014-06-24)
Isotope labeling has revolutionized NMR studies of small nucleic acids, but to extend this technology to larger RNAs, site-specific labeling tools to expedite NMR structural and dynamics studies are required. Using enzymes from the pentose phosphate pathway, we coupled chemically
Anders Virtanen et al.
Critical reviews in biochemistry and molecular biology, 48(2), 192-209 (2013-03-19)
Deadenylation of eukaryotic mRNA is a mechanism critical for mRNA function by influencing mRNA turnover and efficiency of protein synthesis. Here, we review poly(A)-specific ribonuclease (PARN), which is one of the biochemically best characterized deadenylases. PARN is unique among the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service