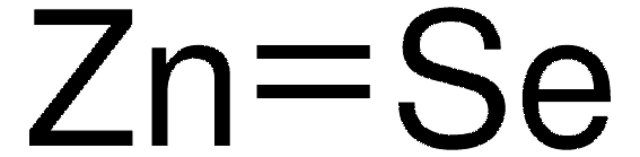14459
Zinc sulfide
purum, 97% (from Zn)
Synonym(s):
Zinc(II) sulfide, Zinc sulphide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ZnS
CAS Number:
Molecular Weight:
97.46
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
97% (from Zn)
reaction suitability
core: zinc
reagent type: catalyst
loss
≤0.5% loss on drying, 105 °C
color
white to faint yellow
density
4.1 g/mL at 25 °C (lit.)
cation traces
Ba: ≤1.5%
Ca: ≤200 mg/kg
Co: ≤500 mg/kg
Fe: ≤100 mg/kg
Mg: ≤500 mg/kg
SMILES string
S=[Zn]
InChI
1S/S.Zn
InChI key
WGPCGCOKHWGKJJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Uses:
- Preparation of flexible transparent conductive coatings essential for fabrication of a variety of printed electronic devices such as flexible displays and solar cells
- Prepare a composite CdS-ZnS/Zirconium-titanium phosphate (ZTP) photocatalyst for hydrogen production under visible light
- Prepare light-controlled bioelectrochemical sensors based on CdSe/ZnS quantum dots
- Catalyst for photocatalytic degradation of organic pollutants
- Preparation of color tunable light-emitting diodes (LEDs)
- Prepare (CdS-ZnS)-TiO2 combined photocatalysts for electricity production via photoelectrocatalysis
- Catalyst for synthesis of spirooxindole derivatives in aqueous medium via Knoevenagel condensation followed by Michael addition
- Prepare CdSe/ZnS q uantum dots for chemiluminescent and chemiluminescence resonance energy transfer (CRET) detection of DNA, metal ions, and aptamer-substrate complexes
- Preparation of ZnS nanocrystals for ultrasensitive protein detection in terms of multiphonon resonance Raman scattering
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ingrid Corazzari et al.
Toxicology in vitro : an international journal published in association with BIBRA, 27(2), 752-759 (2013-01-01)
CdSe Quantum Dots (QDs) are increasingly being employed in both industrial applications and biological imaging, thanks to their numerous advantages over conventional organic and proteic fluorescent markers. On the other hand a growing concern has emerged that toxic elements from
Yi-Chun Yeh et al.
Chemical communications (Cambridge, England), 49(9), 910-912 (2012-12-19)
Functionalization of bacterial cell surfaces has the potential to introduce new activities by chemical modification. Here we show that a bacteriophage-receptor complex can be used to functionalize the surface of two Gram-negative proteobacteria, Escherichia coli and Ralstonia eutropha with CdSe/ZnS
Chi Chen et al.
ACS applied materials & interfaces, 5(3), 1149-1155 (2013-01-18)
Facile aqueous synthesis of near-infrared Ag(2)Te quantum dots (QDs) and Ag(2)Te/ZnS core/shell QDs emitting in the second biological window is reported. The QD synthesis is based on a straightforward cation exchange process between CdTe QDs and Ag(+) ions conducted in
Chang Shu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 104, 143-149 (2012-12-26)
Strong fluorescence and low cytotoxicity ZnSe/ZnS quantum dots (QDs) were synthesized by a facile aqueous phase route. It overcame the defects such as instability and low quantum yield of the quantum dots synthesized by early aqueous phase route. L-Glutathione (GSH)
Hamid Reza Rajabi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 107, 256-262 (2013-02-26)
This work reports a spectrofluorimetric method for selective and sensitive determination of sulfide ion in aqueous solution. The ultra-small zinc sulfide quantum dots (QDs) doped with manganese (ZnS:Mn) were synthesized by using a simple and fast procedure based on the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service