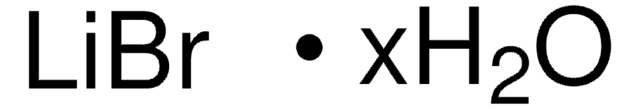All Photos(1)
About This Item
Linear Formula:
LiBr
CAS Number:
Molecular Weight:
86.85
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
form:
liquid
Recommended Products
form
liquid
Quality Level
concentration
49.0-59.0% (silver nitrate titration)
54 wt. % in H2O
density
1.57 g/mL at 25 °C
SMILES string
[Li+].[Br-]
InChI
1S/BrH.Li/h1H;/q;+1/p-1
InChI key
AMXOYNBUYSYVKV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Lithium bromide solution contains 54 wt.% lithium bromide in water. The vapor pressure of the aqueous solution of lithium bromide has been studied over a range of temperature. Lithium bromide solution forms the absorber in the absorption refrigerator. Its pool boiling heat transfer coefficient has been obtained. It is a corrosive medium on metals.
Application
Lithium bromide solution may be used to dissolve/regenerate cellulose to form cellulose gel. It may also be used as a solvent for corrosion studies.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pool boiling performance of lithium bromide solution on enhanced tubes.
Sim YS and Kim NH.
Journal of Mechanical Science and Technology, 29(6), 2555-2563 (2015)
Corrosion behavior and galvanic studies of brass and bronzes in aqueous lithium bromide solutions.
Mu?oz AI, et al.
Corrosion (Houston, TX, United States), 58(7), 560-569 (2002)
Cellulose dissolution in aqueous lithium bromide solutions.
Yang YJ, et al.
Cellulose, 21(3), 1175-1181 (2014)
Thermodynamic properties of aqueous electrolyte solutions. 1. Vapor pressure of aqueous solutions of lithium chloride, lithium bromide, and lithium iodide.
Patil KR, et al.
Journal of Chemical and Engineering Data, 35(2), 166-168 (1990)
Use of differential pulse polarography to study corrosion of galvanized steel in aqueous lithium bromide solution.
Garcia-Anton J, et al.
Corrosion (Houston, TX, United States), 50(2), 91-97 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service