A8681
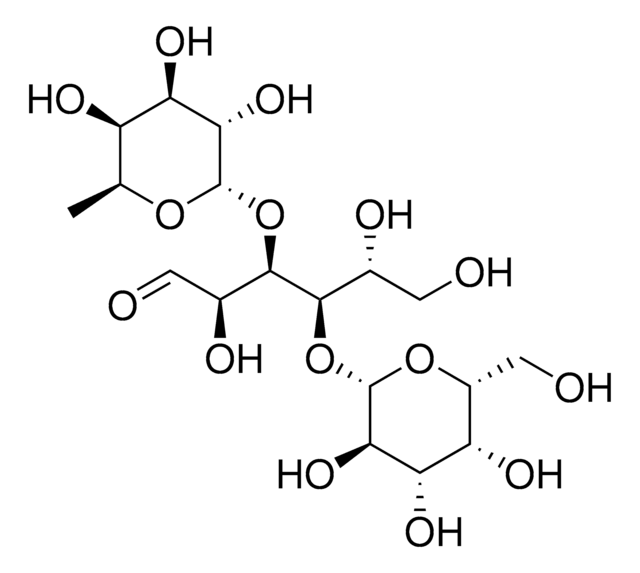
3′-Sialyllactose
from bovine milk or colostrum, ≥97% (HPLC)
Synonym(s):
α-NeuNAc-(2→3)-β-D-Gal-(1→4)-D-Glc, 3′-N-Acetylneuraminyl-D-lactose sodium salt, 3′-SL, 3′-Sialyl-D-lactose, NANA-Lactose
About This Item
Recommended Products
biological source
bovine milk or colostrum
Assay
≥97% (HPLC)
form
powder
color
white
solubility
water: 20 mg/mL, clear, colorless
cation traces
Na: 3.1-4.7%
storage temp.
−20°C
SMILES string
[Na+].[H]C(=O)[C@H](O)[C@@H](O)[C@H](O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O[C@@]2(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O2)[C@H](O)[C@H](O)CO)C([O-])=O)[C@H]1O)[C@H](O)CO
InChI
1S/C23H39NO19.Na/c1-7(29)24-13-8(30)2-23(22(38)39,42-19(13)15(35)10(32)4-26)43-20-16(36)12(6-28)40-21(17(20)37)41-18(11(33)5-27)14(34)9(31)3-25;/h3,8-21,26-28,30-37H,2,4-6H2,1H3,(H,24,29)(H,38,39);/q;+1/p-1/t8-,9-,10+,11+,12+,13+,14+,15+,16-,17+,18+,19+,20-,21-,23-;/m0./s1
InChI key
LTWFUJWFLMHANB-TZCPRLTCSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
O-Linked glycoproteins are usually large proteins with a molecular mass of >200 kDa. Glycosylation generally occurs in high-density clusters and may represent as much as 50-80% of the overall mass.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service