G6008
β-Galactosidase from Escherichia coli
Grade VI, lyophilized powder, ≥250 units/mg protein
Synonym(s):
β-D-Galactoside galactohydrolase, Lactase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Escherichia coli
Quality Level
type
Grade VI
form
lyophilized powder
specific activity
≥250 units/mg protein
mol wt
465 kDa
does not contain
BSA as extender
composition
Protein, ≥50% biuret
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
β-Galactosidase is a tetramer consisting of four equal subunits of 135,000 Da each. It is a sulfhydryl containing enzyme, with 19 cysteine residues per subunit.
Application
β-Galactosidase is conjugated to an antibody that specifically recognizes a target molecule (enzyme immunoassay or EIA). β-Galactosidase is also used as a reporter enzyme to monitor the level of gene expression of a promoter.
Biochem/physiol Actions
β-galactosidase cleaves lactose into its monosaccharide components, glucose and galactose. It also catalyses the transglycosylation of glucose into allolactose, the inducer of β-galactosidase, in a feedback loop.
β-galactosidase cleaves lactose into its monosaccharide components, glucose and galactose. It also catalyses the transglycosylation of glucose into allolactose, the inducer of β-galactosidase, in a feedback loop.
Physical properties
Tetramer molecular weight 465 kDa (subunits 116.3 kDa each)
Unit Definition
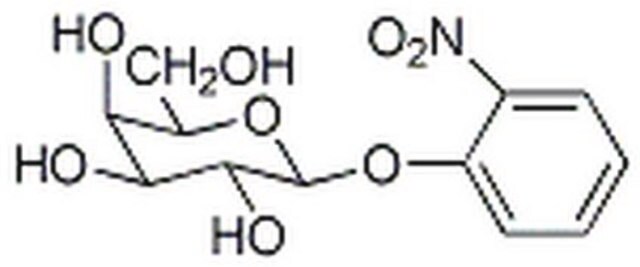
One unit will hydrolyze 1.0 μmole of o-nitrophenyl β-D-galactoside to o-nitrophenol and D-galactose per min at pH 7.3 at 37 °C.
Physical form
Partially purified; contains Tris buffer salts, magnesium chloride, DL-dithiothreitol and 2-mercaptoethanol
inhibitor
Product No.
Description
Pricing
substrate
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
David P Klebl et al.
Frontiers in molecular biosciences, 9, 945772-945772 (2022-08-23)
Advances in single particle cryo-EM data collection and processing have seen a significant rise in its use. However, the influences of the environment generated through grid preparation, by for example interactions of proteins with the air-water interface are poorly understood
PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12.
G R CRAVEN et al.
The Journal of biological chemistry, 240, 2468-2477 (1965-06-01)
K Kato et al.
Journal of immunology (Baltimore, Md. : 1950), 116(6), 1554-1560 (1976-06-01)
1. A method for the conjugation of the Fab' fragment of rabbit IgG with beta-D-galactosidase from Escherichia coli is described. The method consists of two main steps: treatment of the Fab' fragments containing sulfhydryl groups with excess N,N'-o-phenylenedimaleimide, to introduce
Orit Redy-Keisar et al.
Nature protocols, 9(1), 27-36 (2013-12-07)
This protocol describes the synthesis of modular turn-ON QCy7-based probes for the detection of biologically relevant analytes, such as hydrogen peroxide, ubiquitous sulfhydryl and β-galactosidase. The probes presented herein are prepared through a simple procedure that involves the preliminary alkylation
D C Young et al.
Analytical biochemistry, 215(1), 24-30 (1993-11-15)
Bacterial beta-galactosidase is one of several reporter enzymes used in studying the transcriptional activity of eukaryotic promoters. Although it is one of the easiest and least expensive enzymes to assay, its use has been limited because of its low sensitivity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service