About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥99.0%
form
powder
mp
>300 °C (lit.)
solubility
formic acid: water (2:1): 50 mg/mL, clear to slightly hazy, colorless to yellow
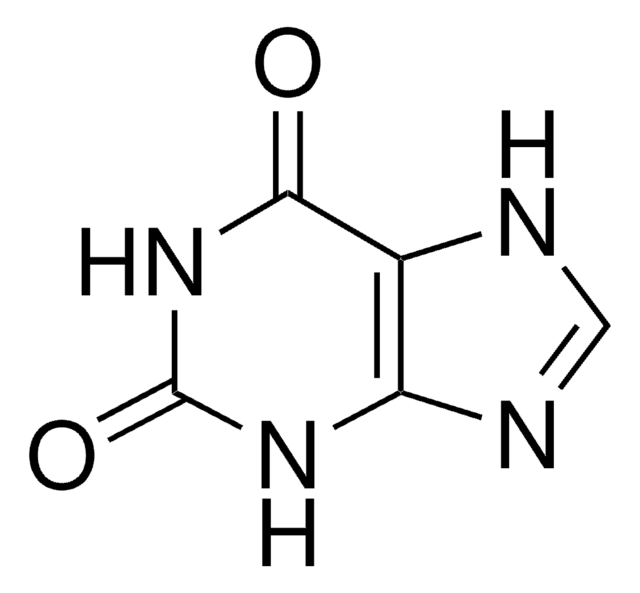
SMILES string
O=C1NC=Nc2nc[nH]c12
InChI
1S/C5H4N4O/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10)
InChI key
FDGQSTZJBFJUBT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- to gavage mice and to evaluate the activity of xanthine oxidase (XO) in pasteurized whole milk in vivo in potassium oxonate (PO) -induced mouse model of hyperuricemia
- as a supplement in Iscove′s modified Dulbecco′s medium (IMDM) medium to cultivate in-vitro cell line (EL-1 cells)
- as a pure standard for the quantification of hypoxanthine in vitreous fluid sample using high-performance liquid chromatography-ultra-violet (HPLC-UV) for the estimation of post-mortem interval
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Uric acid present in animal sera impacts serum-supplemented classical cell culture systems, influencing cell culture performance.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service