L3888
D-Lactic Dehydrogenase from Lactobacillus leichmannii
lyophilized powder, 150-500 units/mg protein
Synonym(s):
(R)-Lactate:NAD+ oxidoreductase, D-LDH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
bacterial (Lactobacillus leichmannii)
Quality Level
form
lyophilized powder
specific activity
150-500 units/mg protein
composition
Protein, ~50% biuret
foreign activity
Malic dehydrogenase <0.5% of base activity
storage temp.
−20°C
General description
Research area: Cell Signaling
Lactate Dehydrogenase (LDH) is classified as an oxidoreductase and is found in various organisms, including both plants and animals. LDH is widely distributed across all tissues, with high concentrations in muscle, kidney, and liver. The genes encoding LDH are LDHA, LDHB, LDHC, and LDHD. The D-isomer is produced by LDHD. There are two types of D-LDHs: NAD-dependent D-LDHs and FAD-dependent D-LDHs.
Lactate Dehydrogenase (LDH) is classified as an oxidoreductase and is found in various organisms, including both plants and animals. LDH is widely distributed across all tissues, with high concentrations in muscle, kidney, and liver. The genes encoding LDH are LDHA, LDHB, LDHC, and LDHD. The D-isomer is produced by LDHD. There are two types of D-LDHs: NAD-dependent D-LDHs and FAD-dependent D-LDHs.
Application
D-Lactic Dehydrogenase from Lactobacillus leichmannii has been used:
- in lactate dehydrogenase activity for testing the chaperone activity of proteins
- to test the kinase activities of purified thiamine monophosphate(ThiM)
- in NADH-coupled steady-state ATPase assay
- to determine cellular lactate
In the food industry, the primary catalysis is coupled to conversion of NADH and H+ to NAD+ with diaphorase coupled with converting the non-fluorescent resazurin to the highly fluorescent substance resorufin to measure the content of D-lactate in food products.
Biochem/physiol Actions
It acts as a crucial checkpoint in gluconeogenesis and DNA metabolism. Elevated levels of LDH in the blood have been observed in various conditions, including heart attacks, cancers, liver disease, muscle trauma, anemia, bone fractures, and infections such as encephalitis, human immunodeficiency virus(HIV), and meningitis. LDH also serves as a non-specific marker of tissue turnover, which is a normal metabolic process. Additionally, reduced D-LDH activity has been found in case of mutations in LDHD found in patients with D-lactic acidosis.
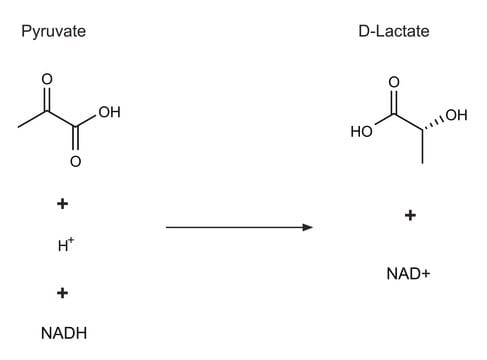
D-lactic dehydrogenase catalyzes the conversion of pyruvate into D-lactate, with oxidation of NADH to NAD+. D-lactic dehydrogenase can also catalyze the reverse reaction, conversion of D-lactate into pyruvate with reduction of NAD+ to NADH.
Unit Definition
D-lactic dehydrogenase catalyzes the conversion of pyruvate into D-lactate, with oxidation of NADH to NAD+. D-lactic dehydrogenase can also catalyze the reverse reaction, conversion of D-lactate into pyruvate with reduction of NAD+ to NADH.
One unit will reduce 1.0 μmole of pyruvate to D-lactate per min at pH 7.0 at 25 °C.
Physical form
Lyophilized powder containing phosphate buffer salts
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lactate dehydrogenase D is a general dehydrogenase for D-2-hydroxyacids and is associated with D-lactic acidosis
Shan J, et al.
Nature Communications, 14, 6638-6638 (2023)
Biochemistry, Lactate Dehydrogenase
Farhana A and Lappin SL
StatPearls [Internet] (2023)
Gerrit Stuivenberg et al.
Journal of food science and technology, 59(9), 3419-3427 (2022-07-26)
Recent studies suggest histamine and d-lactate may negatively impact host health. As excess histamine is deleterious to the host, the identification of bacterial producers has contributed to concerns over the consumption of probiotics or live microorganisms in fermented food items.
Biosynthesis and Activity of Prenylated FMN Cofactors
Wang PH, et al.
Cell Chemical Biology, 25(5), 560-570 (2018)
A Novel Method for Assessing the Chaperone Activity of Proteins
Hristozova N, et al.
PLoS ONE, 11(8), e0161970-e0161970 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service