SAE0159
Microbial Transglutaminase
lyophilized powder, ≥12 units/mg protein
Synonym(s):
Microbial Enzyme, Transglutaminase, Transglutaminase Enzyme
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥12 units/mg protein
shipped in
dry ice
storage temp.
−20°C
General description
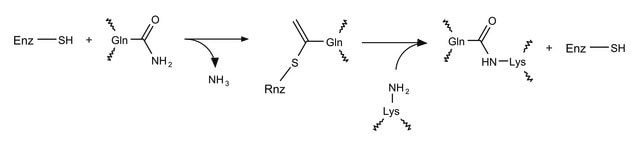
Transglutaminases (TG) are a family of enzymes that catalyze isopeptide bond formation. This bond formation occurs between the γ-carboxyamide group of glutamine and various primary amines (primarily the ε-amino group of lysine). The resulting intermolecular or intramolecular cross-linking of transglutaminase is highly stable and shows high resistance to proteolytic degradation. The transglutaminase crosslinking activity support the formation of blood clots , skin and hair. On the other hand, TG is now being implicated in Celiac disease as well as in Huntington and Parkinson diseases. Historically, Microbial transglutaminase has been heavily used in the food industry. Microbial TG is often preferred for newer applications (such as site-specific protein modification and antibody drug conjugation) due to its small molecular weight and lack of calcium dependency when compared to the mammalian forms.
Application
Microbial Transglutaminase has been used for:
- Protein cross-linking & site-specific labeling
- Antibody Drug Conjugation
- 3D bioprinting bioink preparation
- Food related immunogenicity/pathogenicity related research
Features and Benefits
- Small (~38kDa) and calcium independent enzyme
- Highly purified lyophilized enzyme
- Consistent and reproducible activity
- Characterized for endotoxin content
Preparation Note
For R&D use only. Not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices
Storage and Stability
Store the freeze-dried product at –20 °C. It is recommended to store the reconstituted protein in working aliquots at –20 °C to avoid repeated freeze/ thaw cycles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Matthias Torsten et al.
Frontiers in pediatrics, 6, 389-389 (2019-01-09)
The enzyme microbial transglutaminase is heavily used in the food processing industries to ameliorate food qualities and elongate the products' shelf life. As a protein's glue, it cross-links gliadin peptides, creating neo-complexes that are immunogenic and potentially pathogenic to celiac
Istvan Vermes et al.
Movement disorders : official journal of the Movement Disorder Society, 19(10), 1252-1254 (2004-09-16)
Tissue transglutaminase (tTG) is activated during the apoptotic cell death cascade and plays a key role in the formation of apoptotic bodies. We found significant elevation of tTG concentration in the cerebrospinal fluid (CSF) of 54 patients with Parkinson's disease
Marcela V Karpuj et al.
Neurochemistry international, 40(1), 31-36 (2001-12-12)
Transglutaminase (TGase) activity is increased in affected regions of brains from patients with Huntington's disease (HD). TGase activity is particularly elevated in the nucleus compared with the cytoplasm from these brains. Gamma-glutaminyl-lysyl cross-links have been detected in nuclear inclusions in
Miaomiao Zhou et al.
Biofabrication, 11(2), 025011-025011 (2019-02-12)
Gelatin methacryloyl (GelMA) is a versatile biomaterial that has been shown to possess many advantages such as good biocompatibility, support for cell growth, tunable mechanical properties, photocurable capability, and low material cost. Due to these superior properties, much research has
Martin Griffin et al.
The Biochemical journal, 368(Pt 2), 377-396 (2002-10-09)
Transglutaminases (Tgases) are a widely distributed group of enzymes that catalyse the post-translational modification of proteins by the formation of isopeptide bonds. This occurs either through protein cross-linking via epsilon-(gamma-glutamyl)lysine bonds or through incorporation of primary amines at selected peptide-bound
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service