Derivation of Functional Oligodendrocyte Progenitor Cells (OPCs) from Human Neural Stem Cell Lines
Christine Chen, Michael Moeller, Anna Abai, Nick Asbrock and Vi Chu
MilliporeSigma, Bioscience Division, Temecula, CA, USA
Human neural stem cells (hNSCs) have been intensely studied for their therapeutic potential in neurodegenerative diseases and spinal injuries. Depending on the microenvironment and growth stimuli, these cells can undergo neurogenesis and replace damaged cells. In the central nervous system, neurogenesis alone is not sufficient to repair neuronal damage. Functional neurons need to be protected by a myelin sheath, which is synthesized by oligodendrocytes, in order to successfully deliver a long-distance action potential. Understanding oligodendrocyte development is a necessary milestone towards developing clinical and pharmaceutical treatments for neurodegenerative pathologies such as multiple sclerosis.
In general, hNSCs generate low and inconsistent numbers of oligodendrocytes in vitro. To develop a model system for cultured oligodendrocytes, three neural stem cell lines were tested for their ability to differentiate into oligodendrocyte progenitor cells (OPCs). Two of the cell lines responded to a differentiation regime that included small molecules and growth factors, PDGF, NT3, thyroid hormone (T3) and FGF2. Both cell lines exhibited a 30 to 50% increase in the OPC populations as measured by immunofluorescent staining for multiple oligodendroglial markers: NG2, O4, Sox10 and GalC. These OPCs could be further differentiated to mature oligodendrocytes under mitogen-free conditions. Our data shows that these cells maintain stable OPC characteristics for at least three passages and expressed multiple oligodendrocyte markers upon differentiation. Using a co-culture system, we show that these human OPCs developed into mature oligodendrocytes that produced MBP around the axon in primary rat neuron cultures.
Methods
Human Neural Stem Cell Culture
Two human immortalized neural stem cell lines, ReNcell™ VM (SCC008) and ReNcell™ CX (SCC007) were used along with the ENStem-A™ human ESC-derived neural progenitor cell line (SCR055). Neural stem cells were cultured as monolayers using ReNcell maintenance medium (SCM005) freshly supplemented with 20 ng/mL FGF-2 or ENStem™-A expansion medium (SCM004) freshly supplemented with 20 ng/mL FGF-2 and 2 mM glutamine. ReNcell lines were cultured on 20 μg/mL laminin (L2020) coated flasks while hESC-NPC cells were cultured on Matrigel coated flasks. All cells were maintained in a 5% CO2, 37 °C tissue culture incubator, enzymatically passaged with Accutase® solution (A6964) and seeded at 1X105/cm2 density until they reached 70–80% confluency.
Oligodendrocyte Differentiation and Maturation
Human neural stem cells were harvested and resuspened in their respective growth media at 5 x 105 cells/mL on ultra-low adhesion plates (CLS3471) to form neurospheres. The neurospheres were treated with retinoic acid (R2625) before being switched to oligodendrocyte enrichment medium (OEM: DMEM/F12 with 1X NEAA, 2 mM glutamine, N21 medium supplement, a cocktail of growth factors (thyroid hormone (T3, T5516); recombinant human PDGF-AA (SRP3268), recombinant human neurotrophin-3 (N1905), and recombinant human basic FGF2 (F0291). Neurospheres continued to be cultured in OEM for several passages before being plated on a Matrigel-coated flask for expansion in OPC expansion media (SCM107). Seeding density for enriched OPC cultures was 1–2 x 104 cells/cm2.
Oligodendrocyte Progenitor Cell Characterization
104 cells/cm2 OPCs were plated on 0.1% poly-L-ornithine (P4957) and 10 μg/mL laminin (L2020) coated Millicell® EZ SLIDES (PEZGS0416) or T25 flasks and cultured overnight before switching to growth factor-free medium. Oligodendrocytes were allowed to mature for 2 to 3 weeks before being fixed with 2% paraformaldehyde or lysed for RNA extraction.
Proliferating OPCs were characterized with antibodies specific to early oligodendrocyte markers, including anti-Sox10 (AB5727, 1:200), anti-NG2 (AB5320, 1:200), anti-Olig-2 (AB9610, 1:100), and anti-O4 (MAB345, 1:50) while two-week differentiated oligodendrocytes were characterized with antibodies to intermediate to late oligodendrocyte markers, including anti-GalC (MAB342, 1:200), anti-PLP-1/DM20 (MAB388, 1:100), anti-MBP (05-675, 1:25), anti-MOG (MAB5680, 1:25), anti-CNPase (1:100), and anti-RIP (1:25). Astrocytes were labeled with anti-GFAP (MAB3402, 1:250) and neurons were labeled with anti-bIII tubulin (MAB1637, 1:100) or anti-MAP2 (MAB3418, 1:200).
In Vitro Myelination Assay
12 mm cover glasses were acid-treated with 1N HCl (H1758) overnight, rinsed with Milli-Q® H2O (MILLIQ) and then sterilized with autoclaving. Cover glasses were air dried before being coated with 0.1% poly-L-lysine (P8920) at room temperature for 1 hour and then washed with sterile distilled water. 1 x 105 primary E19 rat hippocampal neurons (R886N-10) were plated on the poly-L-lysine coated cover glass in each well of a 4-well plate in Dulbecco’s Modified Eagle Medium (DMEM) with 10% heat-inactivated horse serum. 24 to 48 hours after plating, cells were transfected with 1 μg of pCAG-GFP plasmid following the manufacturer’s protocol. 24 hours post-transfection, approximately 10–20% of the primary neurons were GFP-positive. Four days after transfection, 2 x 104 human OPCs were seeded to the plate and the neuronal culture medium was replaced with the Human OPC Spontaneous Differentiation Medium (SCM106). Human OPCs and rat primary neuron co-culture was left at 37 °C in a 5% CO2 tissue culture incubator for 21 days with medium changes every 2 to 3 days.
Results
Three neural progenitor cell lines were tested for OPC enrichment. Among them, the ENStem-A ESC-derived NPC line and the immortalized human fetal NSC line (ReNcell VM) contained a significant number of A2B5- and GalC-positive cells in OEM culture. In contrast, the immortalized human cortical NSC line (ReNcell CX) did not demonstrate a significant difference in marker expression between the parental NSC cell line and the directed differentiation conditions (data not shown).
Figure 1 shows the quantitative and representative images of human NSCs cultured in original medium (parental) or in oligodendrocyte enrichment medium (OEM). ENStem- A cells (Figure 1A and B) displayed moderate increases in the O2A progenitor marker, A2B5, and a 3-fold increase in the intermediate oligodendrocyte marker, GalC in OEM culture. In contrast, ReNcell VM cells (Figure 1C and D) cultured in OEM demonstrated more than 6-fold increases in A2B5 expression and less than 2-fold increase in the GalC expression. This illustrates that, even when subjected to the same enrichment procedure, each NSC line responds differently to small molecules and growth factors.

Figure 1. Derived OPC from Human Neural Stem Cell lines. Quantitative measurement of A2B5- or GalC-positive cells in (A) ENStem-A or (C) ReNcell VM-derived cultures in standard medium (parental) or oligodendrocyte-enrichment medium (OEM). Data was calculated based on three randomly selected fields and presented as mean with standard error. (B) and (D) show representative images for (A) and (C). Images were taken with 20X objective. A2B5-Parental (B, D, upper left); A2B5-OEM (B, D, upper right); Gal C-Parental (B, D, bottom left); Gal-C-OEM (B, D, bottom right).
To examine whether OPCs could be stably maintained in oligodendrocyte enrichment medium, we monitored cell proliferation rate by measuring doubling time. Cells doubled every 48 hours for at least 7 weeks in culture (Figure 2A) and the bipolar morphology suggested that these cells were in the neural progenitor/oligodendrocyte progenitor stage of differentiation.
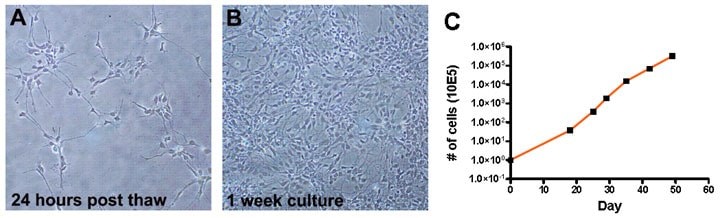
Figure 2. Bright field images of proliferating Human OPCs 24 hours post-thawing (A) and after one week in culture (B). Human OPCs double every 48 hours (C).
We next examined the OPC cells for protein expression by immunofluorescent staining between passage 3 and passage 5. We observed high numbers of cells expressing Olig2 (92%, Figure 3A), Sox10 (81%, Figure 3B), O4 (82%, Figure 3C), NG2 (64%, Figure 3D) and GalC (80%, Figure 3E). After 2 weeks of spontaneous differentiation, cells turned into 50 to 70% of MAP2- or bIII-tubulin-positive neurons (Figure 4) and less than 5% of GFAP-positive astrocytes (Figure 4). 30 to 50% of cells showed robust expression of intermediate to late oligodendrocyte markers MBP, PLP-1, MOG, RIP, GalC and NG2 (Figure 4).

Figure 3. Oligodendrocyte Progenitor Cell Characterization. Human OPCs express early oligodendrocyte markers Olig2, Sox10, O4, NG2 and GalC. Oligodendrocyte progenitor cells were plated at 104 cells/cm2 onto poly-L-ornithine and laminin coated 24 well plates.

Figure 4. Mature Oligodendrocyte Characterization. After 2 weeks of spontaneous differentiation, Human OPCs generate approximately 30% mature oligodendrocytes (MBP, MOG, PLP, RLP, RIP) and ~50% neurons (Tubulin, MAP2). Human OPCs were plated at 104 cells/cm2 onto poly-L-ornithine and laminin coated 24 well plates in Human OPC Expansion Complete Media. Twenty-four hours post-seeding, spontaneous differentiation was initiated by media exchange with Human OPC Spontaneous Differentiation Complete Media.
Rat primary neurons isolated from the embryonic hippocampus are mostly pyramidal neurons that have a distinctive morphology composed of single axons and multiple dendrites. To facilitate identification of the rat primary neurons, green fluorescent protein (GFP) was introduced by transient transfection. 10%-20% cells were successfully transfected and GFP expression was stable for the period of observation. Human ES cell-derived OPCs were added to the rat primary neuronal culture at a ratio of 1:5. Three weeks after initiating coculture, we analyzed the relative degree of myelination by immunofluorescent staining. Myelin basic protein (MBP) is a major component of the myelin sheath. In the absence of Human OPCs, rat primary neuronal cultures exhibited little to no staining for MBP (Figure 5A). In contrast, in the coculture containing GFP-labeled axons and human OPCs, significant MBP staining was observed (Figure 5B). The expression of MBP colocalized with nuclei, providing evidence that MBP is expressed from human OPCs. This data suggested that in the presence of mature or developing axons, human ES cell-derived OPCs can be further maturated to functional oligodendrocytes that are capable of producing myelin to protect the axons.

Figure 5. In-Vitro Myelination Assay. Human ES cell derived OPC’s (Human Nuclei+) myelinate neurons (MBP+) when co-cultured with rat primary neurons (A, B, C).
Conclusions
We have developed a stable stem cell line that can maintain a high percentage of oligodendrocyte progenitors in culture and that could potentially yield functional, myelin-producing oligodendrocytes upon spontaneous differentiation. The cells may also be used to screen for molecules that promote or inhibit human OPC survival and differentiation. Our current efforts are in identifying those molecules that would increase OPC differentiation. This convenient, reliable source of oligodendrocytes may help to advance research into neurodegenerative therapies for diseases such as multiple sclerosis.
GraphQL error: Missing locale!
GraphQL error: Missing locale!
GraphQL error: Missing locale!
FAQ
The cells are provided at what passage?
Cells were banked at passage 3 and can be expanded twice. Cells should not be used beyond passage 6.
How many times can I passage the cells?
Cells can be passaged twice. Upon thawing, cells are at passage 4. With an additional two passages, cells are at passage 6. Cells should not be expanded beyond passage 6.
At what confluency should I passage the cells?
Cells should be passaged when they have reached 80% confluency. It is NOT recommended to passage the cells when they are 100% confluent.
What is the optimal splitting ratio?
The optimal seeding density is 2 to 4 x 104 / cm2. This corresponds to 500,000 cells on an appropriately coated T25 flask or 1:3 to 1:5 split.
Can I freeze my cells?
No, we do not recommend freezing the cells. Performance of cells that have been frozen by individual users can’t be guaranteed.
Can I use my media instead?
The Human OPC Expansion Media was optimized specifically for our Human OPCs. Other media have not been tested. If other culture media are used, cells should be extensively characterized by user to ensure that they retain the correct phenotype and staining characteristics.
Can I use the Human OPC Expansion or Differentiation Media kits to expand, culture and differentiate oligodendrocytes that I have obtained from another vendor or have isolated myself?
No. These media were optimized specifically for our Human OPCs. Other cell types have not been tested. We do not believe that cells obtained from other sources or directly isolated from brain tissues will behave similarly in the media system.
Can I use my own growth factors to differentiate?
Growth factors other than the ones provided in the kit have not been tested. If user is contemplating the use of other growth factors, it is recommended that they use the media kit provided as a positive control to compare effects of other growth factors and cytokines on human OPC expansion and differentiation.
Can I use a different ECM to coat?
ECMs other than the ones outlined in the kit have not been tested. If the user is contemplating the use of other ECMs, it is recommended that they use the reagents and ECMs outlined in kit as a positive control to compare effects of other ECMs on human OPC expansion and differentiation.
To continue reading please sign in or create an account.
Don't Have An Account?