추천 제품
분석
98%
양식
solid
bp
135 °C/18 mmHg (lit.)
mp
48-51 °C (lit.)
작용기
bromo
ketone
phenyl
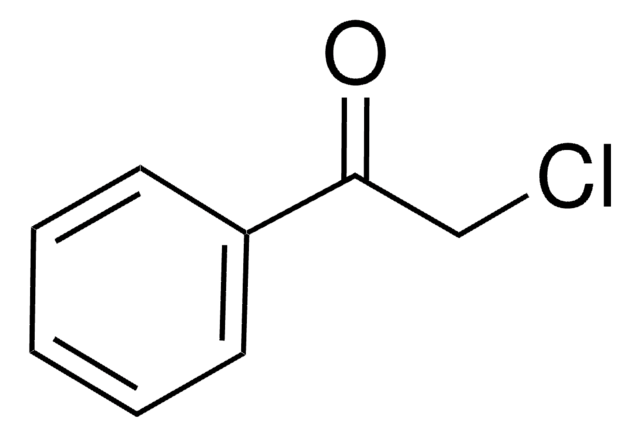
SMILES string
BrCC(=O)c1ccccc1
InChI
1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
InChI key
LIGACIXOYTUXAW-UHFFFAOYSA-N
유전자 정보
human ... PTPN6(5777)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
Effective in key synthesis reactions like Knoevenagel condensation, thalidomide synthesis, and Suzuki coupling for sustainable chemical transformations.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.