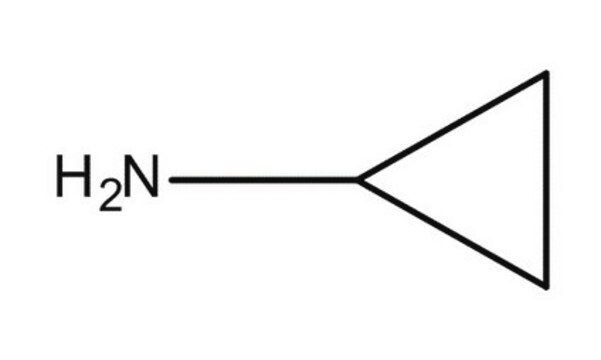
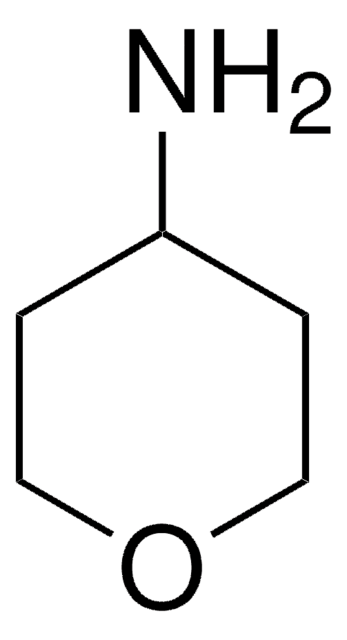
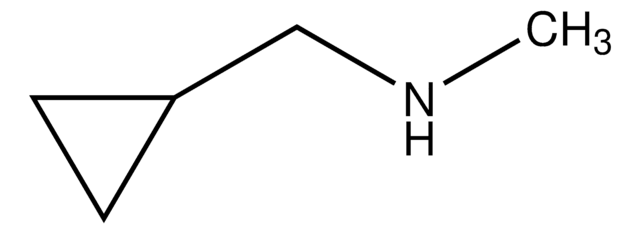
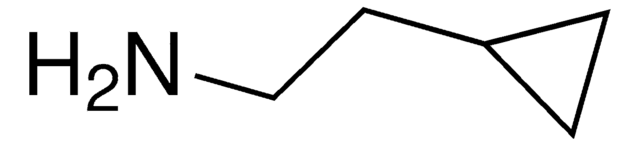
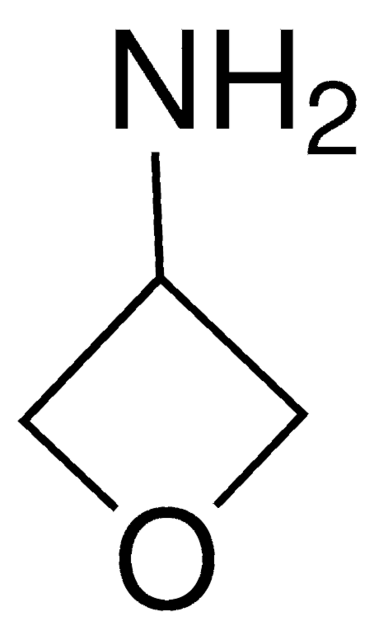
가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
vapor pressure
4.67 psi ( 20 °C)
Quality Level
분석
98%
양식
liquid
autoignition temp.
527 °F
refractive index
n20/D 1.420 (lit.)
bp
49-50 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
SMILES string
NC1CC1
InChI
1S/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
생화학적/생리학적 작용
Cyclopropylamine inactivates cytochrome P450 enzymes by a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme[3]. It is a mechanism-based inhibitor of quinoprotein methylamine dehydrogenase from Paracoccus denitrificans[4].
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
33.8 °F - closed cup
Flash Point (°C)
1 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
Synthesis and Antifungal Activity of 5-[2-(Alkylamino) pyrimidin-4-yl]-4-phenylthiazol-2-cycloalkylamine Derivatives on Phytophthora capsici.
Nam Sw, et al.
J. Korean Chem. Soc., 54(3), 395-402 (2011)
Dapeng Sun et al.
FEBS letters, 517(1-3), 172-174 (2002-06-14)
Cyclopropylamine is a mechanism-based inhibitor of the quinoprotein methylamine dehydrogenase (MADH) from Paracoccus denitrificans. The resulting inactivation is accompanied by the formation of a covalent cross-link between the alpha and beta subunits of MADH. The results of site-directed mutagenesis studies
Jo-Yanne Le Berre et al.
PloS one, 12(12), e0190341-e0190341 (2017-12-28)
Little is known about the responses of plant roots to filamentous pathogens, particularly to oomycetes. To assess the molecular dialog established between the host and the pathogen during early stages of infection, we investigated the overall changes in gene expression
Catherine A Faler et al.
Organic letters, 9(10), 1987-1990 (2007-04-24)
An intermolecular Ti(IV)-mediated cyclopropanation reaction has been used to synthesize substituted 2-phenylcyclopropylamines and constrained analogues of the neurotransmitters histamine and tryptamine. Many hydroxy- and methoxy-substituted phenylcyclopropylamines are known to inhibit monoamine oxidase and have been shown to mimic hallucinogens. These
Christopher L Shaffer et al.
Journal of the American Chemical Society, 124(28), 8268-8274 (2002-07-11)
The role of single electron transfer (SET) in P450-catalyzed N-dealkylation reactions has been studied using the probe substrates N-cyclopropyl-N-methylaniline (2a) and N-(1'-methylcyclopropyl)-N-methylaniline (2b). In earlier work, we showed that SET oxidation of 2a by horseadish peroxidase leads exclusively to products
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.