132004
p-Toluenesulfonyl hydrazide
97%
동의어(들):
p-Toluenesulfonhydrazide, p-Toluenesulfonic acid hydrazide, p-Toluenesulfonyl hydrazide, Tosylhydrazide
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
CH3C6H4SO2NHNH2
CAS Number:
Molecular Weight:
186.23
Beilstein:
610130
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
양식
powder
mp
103-108 °C (lit.)
작용기
hydrazine
SMILES string
Cc1ccc(cc1)S(=O)(=O)NN
InChI
1S/C7H10N2O2S/c1-6-2-4-7(5-3-6)12(10,11)9-8/h2-5,9H,8H2,1H3
InChI key
ICGLPKIVTVWCFT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
p-Toluenesulfonyl hydrazide was used as a reagent for the preparation of tosylhydrazones. It was used to prepare dipyrazolo[1,5-a:4′,3′-c]pyridines and 1,2,3-selenadiazole derivatives.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Self-react. D
보충제 위험성
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Journal of the American Chemical Society, 114, 966-966 (1992)
Mousa Al-Smadi et al.
Molecules (Basel, Switzerland), 13(11), 2740-2749 (2008-11-07)
The commercially available aromatic polyketones 1a-d were utilized for the synthesis of the multi-arm1,2,3-selenadiazole derivatives 3a-d. The preparation starts with the reaction between compounds 1a-d and p-toluenesulfonyl hydrazide to give the corresponding tosylhydrazones 2a-d. Subsequent reaction with selenium dioxide leads
Dipyrazolo[1,5-a:4',3'-c]pyridines - a new heterocyclic system accessed via multicomponent reaction.
Wolfgang Holzer et al.
Beilstein journal of organic chemistry, 8, 2223-2229 (2013-02-01)
The synthesis of dipyrazolo[1,5-a:4',3'-c]pyridines is described. Easily obtainable 5-alkynylpyrazole-4-carbaldehydes, p-toluenesulfonyl hydrazide, and an aldehyde or ketone containing an α-hydrogen atom were reacted in a silver triflate catalyzed multicomponent reaction affording new tricyclic compounds with a dipyrazolo[1,5-a:4',3'-c]pyridine core. Detailed NMR spectroscopic
Journal of the American Chemical Society, 115, 2473-2473 (1993)
Anna V Gudmundsdottir et al.
Organic letters, 10(16), 3461-3463 (2008-07-12)
N'-Glycopyranosylsulfonohydrazides are introduced as glycosyl donors for protecting group free synthesis of O-glycosides, glycosyl azides, and oxazolines. Mono- and disaccharides containing a reducing terminal N-acetylglucosamine residue were condensed with p-toluenesulfonylhydrazide to give the desired beta- d-pyranose donors. These donors can
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.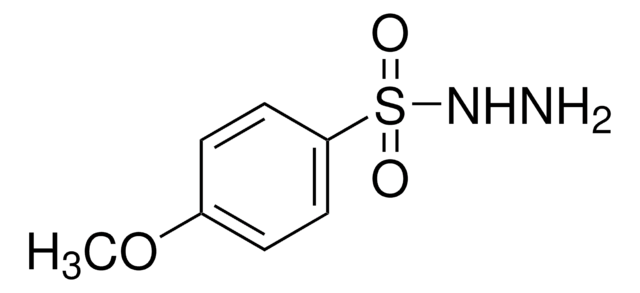
![1H-1,2,3-Triazolo[4,5-b]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/344/744/1e7fa2cf-1258-48e0-909f-92509981f43d/640/1e7fa2cf-1258-48e0-909f-92509981f43d.png)









