추천 제품
분석
≥93% (GC)
광학 활성
[α]/D -58±5°, neat
refractive index
n20/D 1.496
작용기
hydroxyl
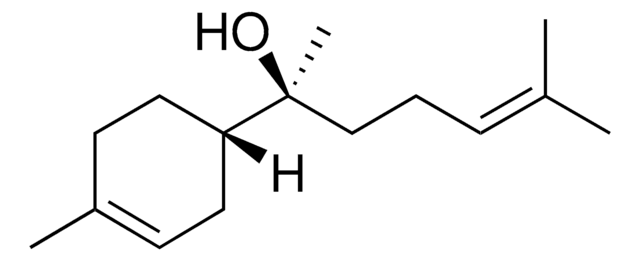
SMILES string
C\C(C)=C\CC[C@](C)(O)[C@H]1CCC(C)=CC1
InChI
1S/C15H26O/c1-12(2)6-5-11-15(4,16)14-9-7-13(3)8-10-14/h6-7,14,16H,5,8-11H2,1-4H3/t14-,15+/m1/s1
InChI key
RGZSQWQPBWRIAQ-CABCVRRESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
생화학적/생리학적 작용
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
289.4 °F - closed cup
Flash Point (°C)
143 °C - closed cup
개인 보호 장비
Eyeshields, Gloves
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.