408484
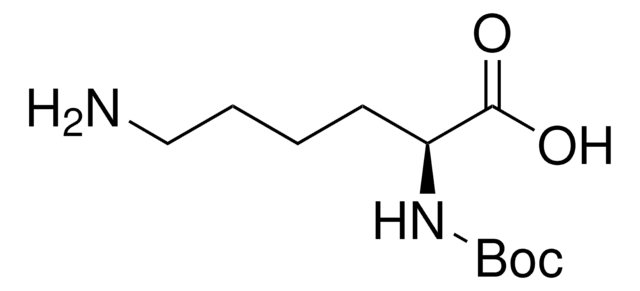
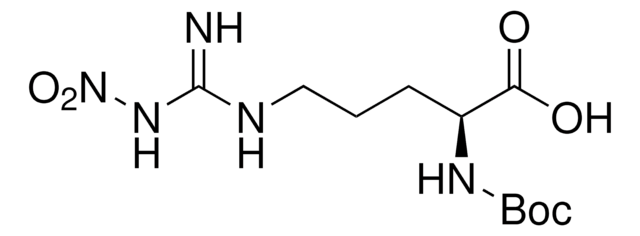
Boc-Arg-OH
for peptide synthesis
동의어(들):
Nα-(tert-Butoxycarbonyl)-L-arginine, Nα-Boc-L-arginine
About This Item
추천 제품
제품명
Boc-Arg-OH,
양식
powder
광학 활성
[α]20/D −6.5°, c = 1 in acetic acid
반응 적합성
reaction type: Boc solid-phase peptide synthesis
불순물
5-10% n-butanol
응용 분야
peptide synthesis
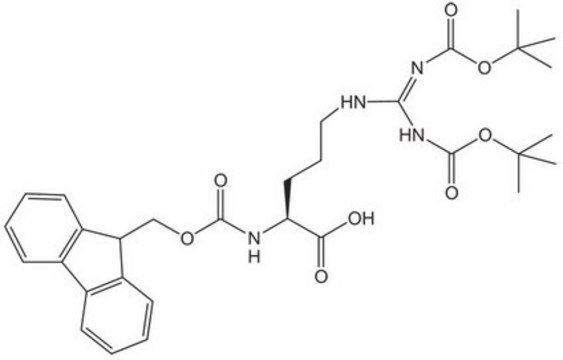
SMILES string
CC(C)(C)OC(=O)N[C@@H](CCCNC(N)=N)C(O)=O
InChI
1S/C11H22N4O4/c1-11(2,3)19-10(18)15-7(8(16)17)5-4-6-14-9(12)13/h7H,4-6H2,1-3H3,(H,15,18)(H,16,17)(H4,12,13,14)/t7-/m0/s1
InChI key
HSQIYOPBCOPMSS-ZETCQYMHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Studying the kinetics of tanning reactions: The study compares the color development kinetics in tanning reactions involving dihydroxyacetone with both free and Boc-protected basic amino acids, highlighting Boc-Arg-OH′s role in stabilizing reactive intermediates in cosmetic chemistry (Sun et al., 2022).
- Protonation states in peptide synthesis: This research examines the side-chain protonation states of a fluorescent arginine derivative, including Boc-Arg-OH, to optimize fluorescence in peptide synthesis for bioimaging applications (Marshall et al., 2019).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.