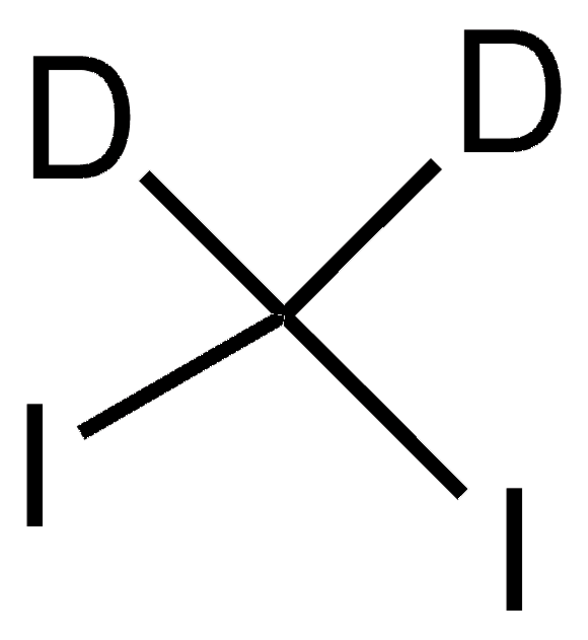
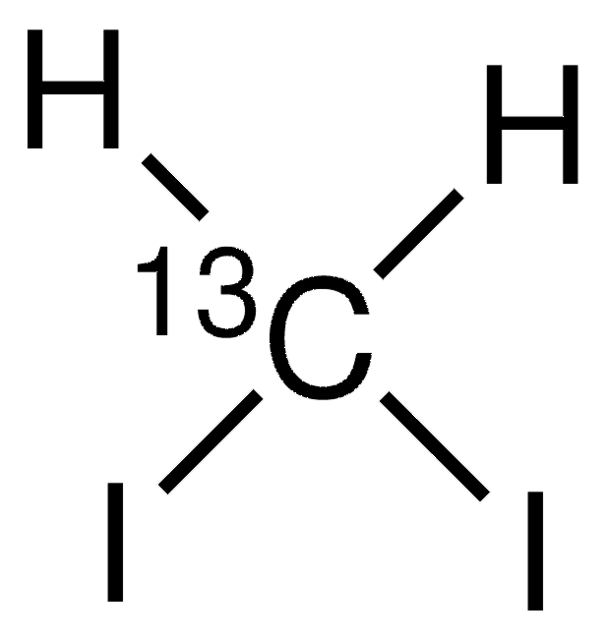
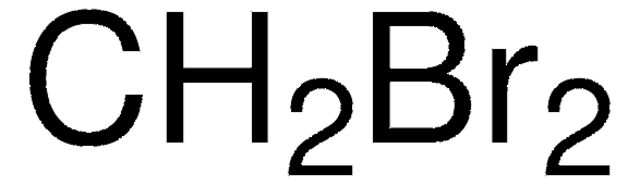추천 제품
vapor density
6.05 (vs air)
Quality Level
vapor pressure
34.9 mmHg ( 20 °C)
분석
99%
양식
liquid
refractive index
n20/D 1.541 (lit.)
bp
96-98 °C (lit.)
mp
−52 °C (lit.)
density
2.477 g/mL at 25 °C (lit.)
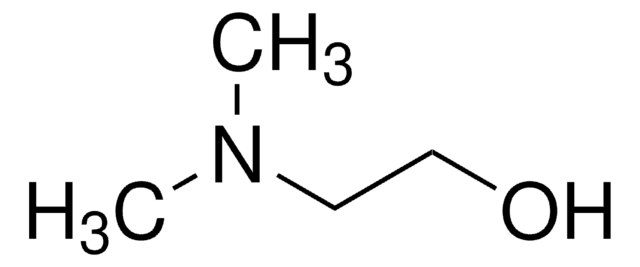
SMILES string
BrCBr
InChI
1S/CH2Br2/c2-1-3/h1H2
InChI key
FJBFPHVGVWTDIP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Dibromomethane may be used for the cyclopropanation of alkenes via Simmons-Smith type reaction. It may also be used in a novel methodology for the preparation of reaction intermediates such as α-substituted acroleins, α-methylene esters, α-keto esters, α-methylene lactones, α-methylene lactams, and α-keto lactones.
관련 제품
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Cyclopropanations of alkenes using dibromomethane.
Friedrich E
The Journal of Organic Chemistry, 50(23), 4640-4642 (1985)
Dibromomethane as one-carbon source in organic synthesis: a versatile methodology to prepare the cyclic and acyclic ?-methylene or ?-keto acid derivatives from the corresponding terminal alkenes.
Hon Y, et. Al.
Tetrahedron, 60(22), 4837-4860 (2004)
Dibromomethane as one-carbon source in organic synthesis: microwave-accelerated ?-methylenation of ketones with dibromomethane and diethylamine.
Hon Y, et. Al.
Tetrahedron, 59(9), 1509-1520 (2003)
Dibromomethane as one-carbon source in organic synthesis: total synthesis of (?)-and (?)-methylenolactocin.
Hon Y, et. Al.
Tetrahedron, 61(10), 2713-2723 (2005)
Cyclopropanes from an Easily Prepared, Highly Active Zinc?Copper Couple, Dibromomethane, and Olefins.
LeGoff E
The Journal of Organic Chemistry, 29(7), 2048-2050 (1964)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.