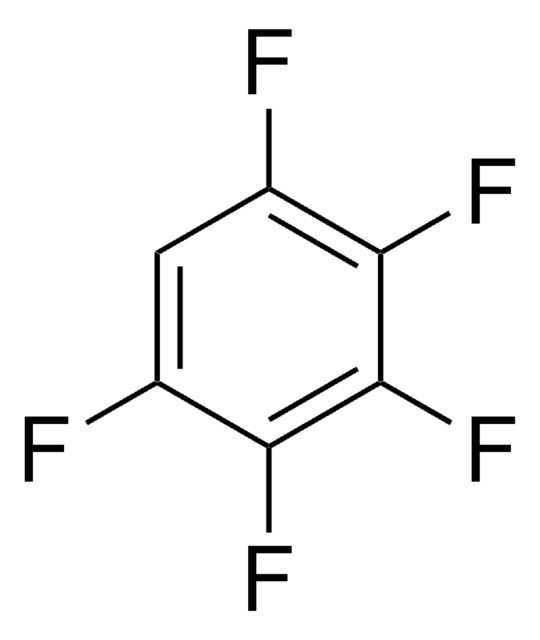
추천 제품
분석
99%
양식
liquid
refractive index
n20/D 1.377 (lit.)
bp
80-82 °C (lit.)
mp
3.7-4.1 °C (lit.)
density
1.612 g/mL at 25 °C (lit.)
SMILES string
Fc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F6/c7-1-2(8)4(10)6(12)5(11)3(1)9
InChI key
ZQBFAOFFOQMSGJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Hexafluorobenzene can react with:
It can be used:
- Ethyl magnesium bromide in the presence of transition metal halides to form the corresponding perfluoroarylmagnesium compound that can undergo Grignard reactions.
- The sodium salt of the appropriate phenol in 1,3-dimethyl-2-imidazolidinone (DMEU) to form the corresponding hexakis(aryloxy)benzenes.
It can be used:
- As a ligand to synthesize novel ruthenium(0) and osmium(0) hexafluorobenzene complexes.
- As a solvent and promoter for the ring-closing metathesis (RCM) to form tetrasubstituted olefins in the presence of a ruthenium-based catalyst.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
50.0 °F - closed cup
Flash Point (°C)
10 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis of hexakis (aryloxy) benzenes: x-ray analysis of hexakis (phenyloxy) benzene and of the acetonitrile clathrate of hexakis (3, 5-dimethylphenyloxy) benzene
Gilmore C J, et al.
Tetrahedron Letters, 24(31), 3269-3272 (1983)
Synthesis of new ? 4-hexafluorobenzene complexes of ruthenium and osmium from atoms of the metals: crystal structure of [Ru (? 6-C 6 H 3 Me 3-1, 3, 5)(? 4-C 6 F 6)]
Martin A, et al.
Journal of the Chemical Society, (15), 2251-2255 (1994)
Markus Allesch et al.
The journal of physical chemistry. B, 111(5), 1081-1089 (2007-02-03)
We report on the aqueous hydration of benzene and hexafluorobenzene, as obtained by carrying out extensive (>100 ps) first principles molecular dynamics simulations. Our results show that benzene and hexafluorobenzene do not behave as ordinary hydrophobic solutes, but rather present
M Albertí et al.
The journal of physical chemistry. A, 115(40), 10871-10879 (2011-09-03)
The effect of some leading intermolecular interaction components on specific features of weakly bound clusters involving an aromatic molecule, a closed shell ion, and Ar atoms is analyzed by performing molecular dynamics simulations on potential energy surfaces properly formulated in
Mariana Palma et al.
Frontiers in physiology, 11, 205-205 (2020-04-09)
Practical diets for commercial barramundi production rarely contain greater than 10% starch, used mainly as a binding agent during extrusion. Alternative ingredients such as digestible starch have shown some capacity to spare dietary protein catabolism to generate glucose. In the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.