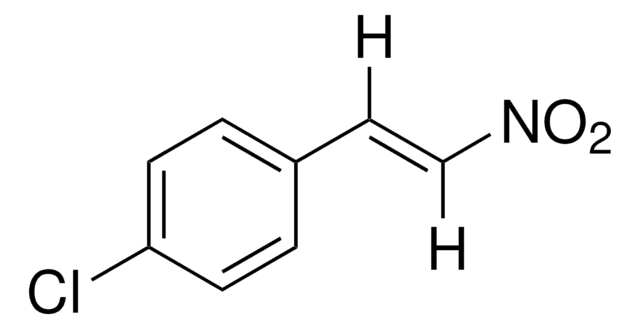
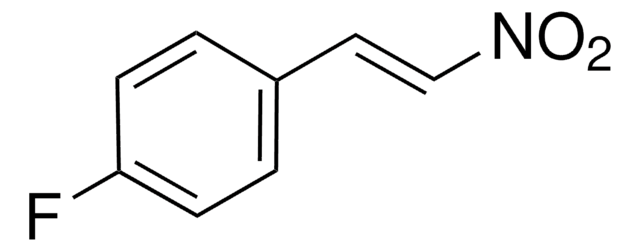
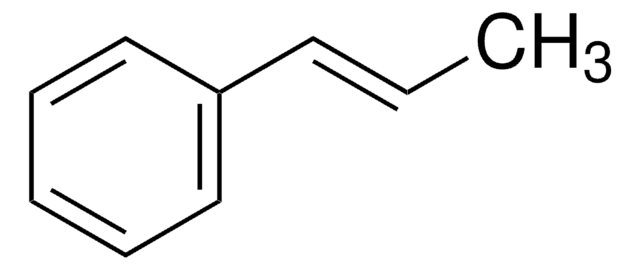
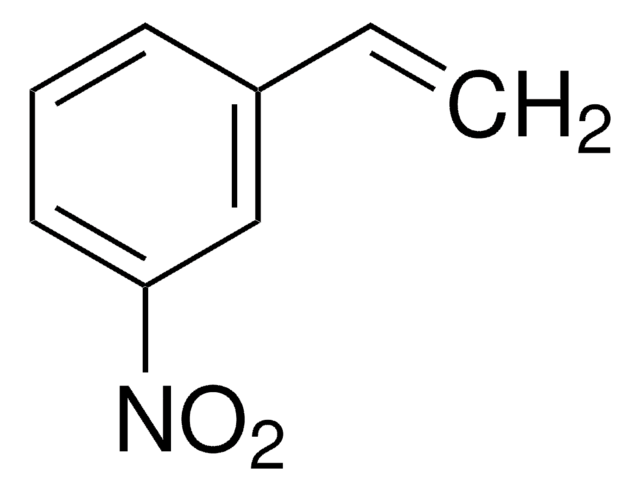
추천 제품
Quality Level
분석
99%
양식
crystals
bp
250-260 °C (lit.)
mp
55-58 °C (lit.)
저장 온도
2-8°C
SMILES string
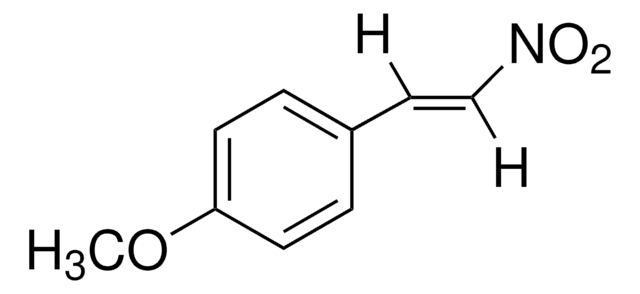
[O-][N+](=O)\C=C\c1ccccc1
InChI
1S/C8H7NO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
PIAOLBVUVDXHHL-VOTSOKGWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Bridging between organocatalysis and biocatalysis: asymmetric addition of acetaldehyde to β-nitrostyrenes catalyzed by a promiscuous proline-based tautomerase.
Ellen Zandvoort et al.
Angewandte Chemie (International ed. in English), 51(5), 1240-1243 (2011-12-23)
Yan Huang et al.
The Journal of organic chemistry, 74(3), 1252-1258 (2008-12-31)
A convenient and efficient way for the highly diastereoselective synthesis of beta-substituted-alpha,gamma-diaminobutyric acids and pyrrolidines containing multichiral centers has been well-developed. Michael addition of chiral tricyclic iminolactones 1 and 2 to nitroalkenes afforded the adducts in good yields (up to
Hui Yang et al.
Organic & biomolecular chemistry, 10(16), 3229-3235 (2012-03-10)
(S)-Proline-catalyzed nitro-Michael additions of aldehydes and ketones to β-nitrostyrene were investigated computationally (MP2/6-311+G**//M06-2X/6-31G**). Contrary to what is usually assumed in organocatalysis, the lowest-energy transition states of proline-catalyzed nitro-Michael reactions do not necessarily involve the carboxylic acid group of the proline
Kamal Nain Singh et al.
Bioorganic & medicinal chemistry letters, 22(13), 4225-4228 (2012-06-08)
An efficient asymmetric Michael addition of cyclic ketones to β-nitrostyrenes using secondary diamine as an organocatalyst derived from l-proline and (R)-α-methylbenzyl amine has been described. This pyrrolidine based catalyst 1 was found to be very effective to synthesize various γ-nitrocarbonyl
Catalytic asymmetric intermolecular Stetter reactions of enolizable aldehydes with nitrostyrenes: computational study provides insight into the success of the catalyst.
Daniel A DiRocco et al.
Angewandte Chemie (International ed. in English), 51(10), 2391-2394 (2012-01-28)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.