추천 제품
일반 설명
A quantitative assay for tumor transendothelial migration has been described using a modified Boyden chamber system, (Okada, 1994; Li, 1999; Laferriere, 2001). The Boyden Chamber system is a two-chamber system with a porous membrane providing an interface between these two chambers. Endothelial cells are cultured on top of the porous membrane that is coated with an extracelluar matrix (ECM) protein. A tumor cell suspension is added above the endothelial monolayer. The invasion of tumor cells across the endothelium is determined by measuring the number of cells that migrate to the lower chamber.
Millipore′s QCM Tumor Cell Transendothelial Cell Migration Assay - Colorimetric provides an efficient model to analyze the ability of tumor cells to invade the endothelium. The assay is designed with an 8 μm pore size cell culture insert, appropriate for most cancer cell lines. The upper side of the cell culture insert is coated with fibronectin to support the optimal attachment and growth of endothelial cells. The assay allows investigators to compare the invasiveness of a variety of tumor cell lines, and to evaluate the effects of various factors influencing the process.
Precoated cell culture inserts are provided in the Millipore QCM Tumor Cell Transendothelial Cell Migration Assay to significantly reduce assay time. Additionally, the assay allows quantitative analysis of tumor cell migration. Following incubation of tumor cells with the endothelial cell layer, invasive tumor cells are stained and quantified. In a departure from traditional Boyden methodology, stain is eluted with extraction buffer, transferred to a microplate, and measured spectrophotometrically. (Prior to elution, the investigator has the option of counting cells individually, if desired.) Spectrophotometric absorbance correlates with cell migration.
Millipore′s QCM Tumor Cell Transendothelial Cell Migration Assay - Colorimetric provides an efficient model to analyze the ability of tumor cells to invade the endothelium. The assay is designed with an 8 μm pore size cell culture insert, appropriate for most cancer cell lines. The upper side of the cell culture insert is coated with fibronectin to support the optimal attachment and growth of endothelial cells. The assay allows investigators to compare the invasiveness of a variety of tumor cell lines, and to evaluate the effects of various factors influencing the process.
Precoated cell culture inserts are provided in the Millipore QCM Tumor Cell Transendothelial Cell Migration Assay to significantly reduce assay time. Additionally, the assay allows quantitative analysis of tumor cell migration. Following incubation of tumor cells with the endothelial cell layer, invasive tumor cells are stained and quantified. In a departure from traditional Boyden methodology, stain is eluted with extraction buffer, transferred to a microplate, and measured spectrophotometrically. (Prior to elution, the investigator has the option of counting cells individually, if desired.) Spectrophotometric absorbance correlates with cell migration.
Tumor metastasis is a multistep cascade. In order for tumor cells to migrate from a primary tumor mass to distant locations, they must invade through the basal membrane and into blood vessels (intravasation), circulate in the blood stream, survive during transport, then migrate out of a blood vessel (extravasation) to establish micrometastases. The penetration of circulating tumor cells into the endothelium is a crucial step for tumor metastasis.
애플리케이션
Calculation of Results
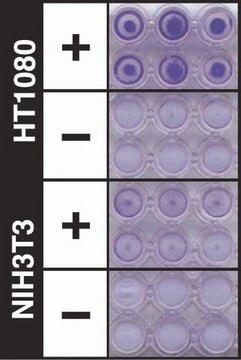
Results of the assay may be illustrated graphically using a bar chart to display optical density (OD) values at ~570 nm.
A typical tumor cell transendothelial migration experiment will compare with a negative control. Negative control chambers, containing HUVECs only, function to determine the level of HUVECs migrating through the membrane. Cell migration in these chambers is generally low as there is no ECM on the lower side of the membrane to support endothelial migration, and these chambers are typically used as "blanks" for interpretation of data. As such, migration in test wells can be described as the value of tumor transendothelial migration minus the amount of migration visualized in the HUVEC only control.
Additional migration may also be induced or inhibited in test wells through the addition of cytokines or other pharmacological agents.
The following figures demonstrate typical migration results. One should use the data at left for reference only. This data should not be used to interpret actual assay results.
Results of the assay may be illustrated graphically using a bar chart to display optical density (OD) values at ~570 nm.
A typical tumor cell transendothelial migration experiment will compare with a negative control. Negative control chambers, containing HUVECs only, function to determine the level of HUVECs migrating through the membrane. Cell migration in these chambers is generally low as there is no ECM on the lower side of the membrane to support endothelial migration, and these chambers are typically used as "blanks" for interpretation of data. As such, migration in test wells can be described as the value of tumor transendothelial migration minus the amount of migration visualized in the HUVEC only control.
Additional migration may also be induced or inhibited in test wells through the addition of cytokines or other pharmacological agents.
The following figures demonstrate typical migration results. One should use the data at left for reference only. This data should not be used to interpret actual assay results.
Research Category
Apoptosis & Cancer
Apoptosis & Cancer
This QCM Tumor Cell Transendothelial Cell Migration Assay -Colorimetric provides an efficient model to analyze the ability of tumor cells to invade the endothelium.
포장
24 Assays
성분
1. Migration Test Plate: (Part No. CS202627) Two 24-well culture plates, each containing 12 human fibronectin-coated cell culture inserts (8 μm pore size), sufficient for the evaluation of 24 test samples
2. TNFα: (Part No. 2004167) One tube - 20 μL at 0.1 mg/mL.
3. Cell Stain Solution*: (Part No. 20294) One vial - 10 mL
4. Extraction Buffer: (Part No. 20295) One vial - 10 mL
5. 24 well Stain Extraction Plate: (Part No. 2005871) One plate.
6. 96 well Stain Quantitation Plate: (Part No. 2005870) One plate.
7. Swabs: (Part No. 10202) 50 each.
8. Forceps: (Part No. 10203) 1 pair.
2. TNFα: (Part No. 2004167) One tube - 20 μL at 0.1 mg/mL.
3. Cell Stain Solution*: (Part No. 20294) One vial - 10 mL
4. Extraction Buffer: (Part No. 20295) One vial - 10 mL
5. 24 well Stain Extraction Plate: (Part No. 2005871) One plate.
6. 96 well Stain Quantitation Plate: (Part No. 2005870) One plate.
7. Swabs: (Part No. 10202) 50 each.
8. Forceps: (Part No. 10203) 1 pair.
저장 및 안정성
TNFα should be stored at -20°C. All other components should be store at 2° to 8°C. Please refer to kit label for expiration date.
법적 정보
Accutase is a registered trademark of Innovative Cell Technologies, Inc.
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
면책조항
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Dorota Gil et al.
Cellular signalling, 72, 109642-109642 (2020-04-20)
Malignant transformation is characterized by a phenotype "switch" from E- to N-cadherin - a major hallmark of epithelial to mesenchymal transition (EMT). The increased expression of N-cadherin is commonly followed by a growing capacity for migration as well as resistance
문서
Cell based angiogenesis assays to analyze new blood vessel formation for applications of cancer research, tissue regeneration and vascular biology.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.