추천 제품
Quality Level
분석
≥97.0% (TLC)
양식
powder
mp
240-241 °C (lit.)
작용기
amine
oxime
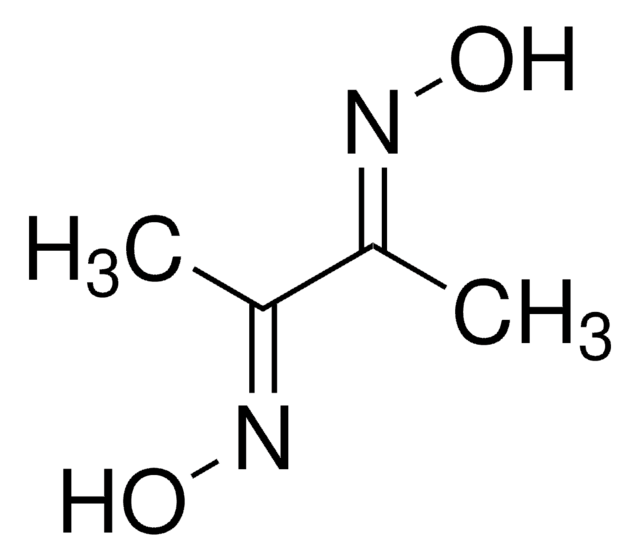
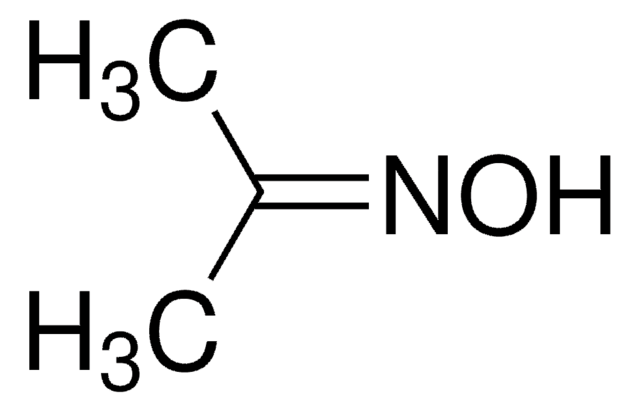
SMILES string
CC(=N/O)\C(C)=N\O
InChI
1S/C4H8N2O2/c1-3(5-7)4(2)6-8/h7-8H,1-2H3/b5-3+,6-4+
InChI key
JGUQDUKBUKFFRO-GGWOSOGESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Dimethylglyoxime can be used:
- As a ligand for the synthesis of cobaloxime complexes which are used as electrocatalysts for the production of hydrogen by electroreduction of protons.
- As a precipitant for the synthesis of NiO and CuI nanoparticles.P
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Synthesis and characteristics of NiO nanoparticles by thermal decomposition of nickel dimethylglyoximate rods.
Li X, et al.
Solid State Communications, 137(11), 581-584 (2006)
Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases.
Razavet M, et al.
Inorganic Chemistry, 44(13), 4786-4795 (2005)
Carsten R Hamann et al.
Contact dermatitis, 68(1), 15-22 (2012-12-12)
Nickel is widely used in coins; nickel may cause contact allergy and allergic contact dermatitis in those who handle them. To investigate alloy use, coin composition and nickel and cobalt release for a worldwide selection of currently circulating coins. Eight
Jacob P Thyssen et al.
The Science of the total environment, 409(22), 4663-4666 (2011-09-06)
Nickel and cobalt allergy remain frequent in dermatitis patients. It is important to determine possible nickel and cobalt exposures at work as these may offer important information to regulators and physicians who perform patch testing. Clinical relevance of metal exposure
Dilek Bakircioglu
Environmental science and pollution research international, 19(6), 2428-2437 (2012-01-26)
An online cloud-point extraction (CPE) coupled with flow injection method is developed for the separation and preconcentration of palladium and lead from various matrices using flame atomic absorption spectrometry (FAAS). The method employs the formation of complexes of the metallic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.