46802
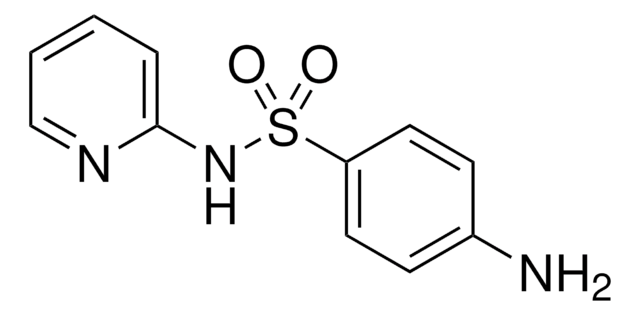
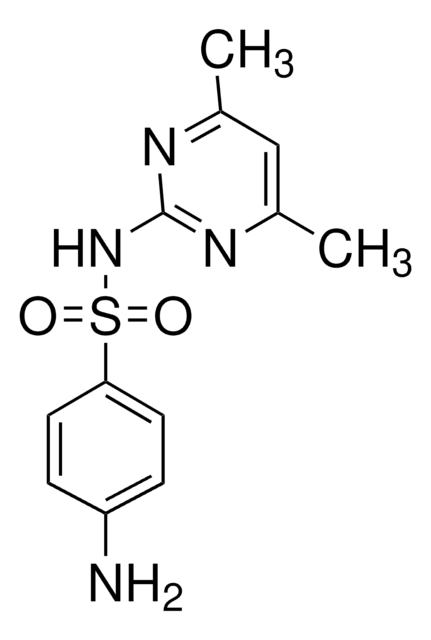
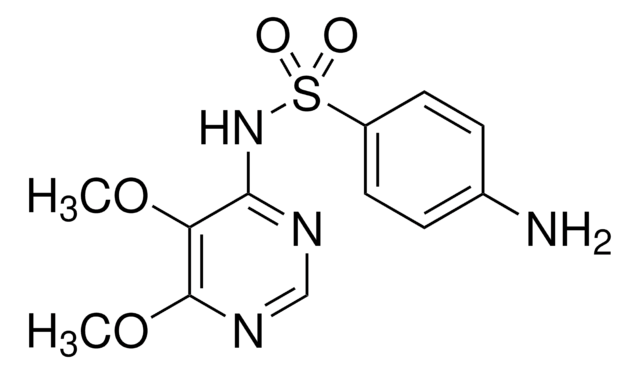
Sulfamethazine
VETRANAL®, analytical standard
동의어(들):
4,6-Dimethylsulfadiazine, 4-Amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide, Sulfadimethyldiazine, Sulfadimidine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C12H14N4O2S
CAS Number:
Molecular Weight:
278.33
Beilstein:
261304
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
Agency
EPA 1694
제품 라인
VETRANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
항생제 활성 스펙트럼
Gram-negative bacteria
Gram-positive bacteria
응용 분야
clinical testing
형식
neat
동작 모드
DNA synthesis | interferes
enzyme | inhibits
SMILES string
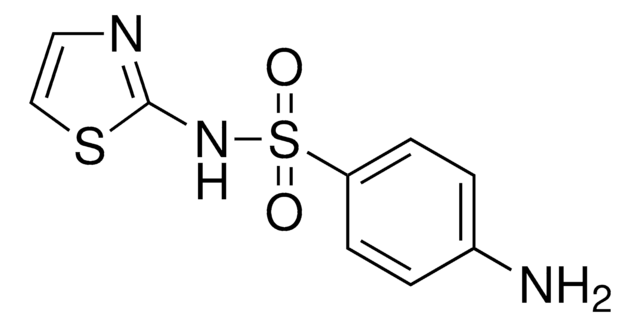
Cc1cc(C)nc(NS(=O)(=O)c2ccc(N)cc2)n1
InChI
1S/C12H14N4O2S/c1-8-7-9(2)15-12(14-8)16-19(17,18)11-5-3-10(13)4-6-11/h3-7H,13H2,1-2H3,(H,14,15,16)
InChI key
ASWVTGNCAZCNNR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: sulfonamide
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
생화학적/생리학적 작용
An antimicrobial sulfur drug. Induces CYP3A4 expression and acetylated by N-acetyltransferase. Exhibits sex dependent pharmacokinetics, metabolized by the male specific isoform CYP2C11.
법적 정보
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Vincent Curtin et al.
Molecular pharmaceutics, 10(1), 386-396 (2012-11-29)
The coprocessing of active pharmaceutical ingredient (API) with an excipient which has a high glass transition temperature (T(g)) is a recognized strategy to stabilize the amorphous form of a drug. This work investigates whether coprocessing a model API, sulfadimidine (SDM)
Ma Jesús García-Galán et al.
The Science of the total environment, 409(24), 5505-5512 (2011-09-29)
Degradation of the sulfonamide sulfamethazine (SMZ) by the white-rot fungus Trametes versicolor was assessed. Elimination was achieved to nearly undetectable levels after 20 h in liquid medium when SMZ was added at 9 mg L(-1). Experiments with purified laccase and
M Rérat et al.
Preventive veterinary medicine, 103(4), 265-273 (2011-09-29)
The present study was conducted to evaluate the efficacy of two prophylactic antibiotic treatments against bovine respiratory disease (BRD) in veal calves. In addition, the antibiotic susceptibilities of isolated Pasteurellaceae were tested. The calves were treated either on the day
Marc Teixidó et al.
Environmental science & technology, 45(23), 10020-10027 (2011-10-27)
Adsorption of ionizable compounds by black carbon is poorly characterized. Adsorption of the veterinary antibiotic sulfamethazine (SMT; a.k.a., sulfadimidine; pK(a1) = 2.28, pK(a2) = 7.42) on a charcoal was determined as a function of concentration, pH, inorganic ions, and organic
Juan Gao et al.
Environmental science & technology, 46(5), 2642-2651 (2012-01-17)
The transformation of the sulfonamide antimicrobial sulfamethazine (SMZ) by a synthetic analogue of the birnessite-family mineral vernadite (δ-MnO(2)) was studied. The observed pseudo-first-order reaction constants (k(obs)) decreased as the pH increased from 4.0 to 5.6, consistent with the decline in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.