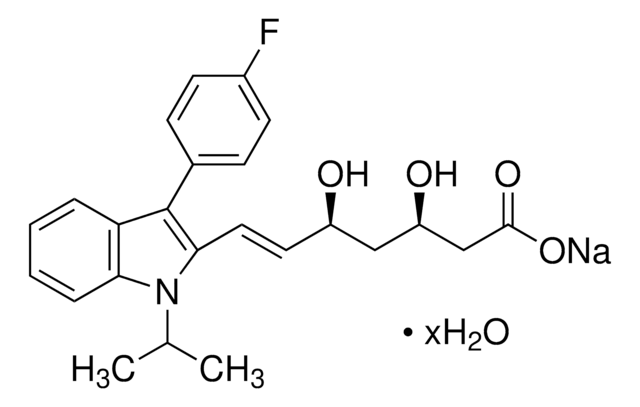
F8682
Fosmidomycin sodium salt hydrate
≥95% (NMR)
동의어(들):
(3-(Formylhydroxyamino)propyl)phosphonic acid sodium salt, (3-(N-Hydroxyformamido)propyl)phosphonic acid sodium salt, FR 31564
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C4H10NO5P · xNa+ · yH2O
CAS Number:
Molecular Weight:
183.10 (anhydrous free acid basis)
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.77
추천 제품
Quality Level
분석
≥95% (NMR)
양식
powder
저장 조건
desiccated
색상
white to beige
solubility
H2O: 20 mg/mL, clear
저장 온도
−20°C
SMILES string
[P](=O)(O)(O)CCCN(O)C=O
InChI
1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10)
InChI key
GJXWDTUCERCKIX-UHFFFAOYSA-N
애플리케이션
Fosmidomycin sodium salt hydrate has been used as an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in a study to determine monotropin carvacrol biosynthesis in Satureja khuzistanica plant.
생화학적/생리학적 작용
Fosmidomycin is an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) (MEP synthase): an antimalarial compound.
Fosmidomycin is an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) (MEP synthase): an antimalarial compound. 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) is an enzyme involved in the first step in the nonmevalonate pathway for isoprenoid biosynthesis in Gram-negative, Gram-positive bacteria, plants, and the parasite causing the most virulent form of malaria, Plasmodium falciparum (Mammals produce isoprenoids via the mevalonate pathway).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Miguel Lanaspa et al.
Antimicrobial agents and chemotherapy, 56(6), 2923-2928 (2012-03-21)
The combination of fosmidomycin and clindamycin (F/C) is effective in adults and older children for the treatment of malaria and could be an important alternative to existing artemisinin-based combinations (ACTs) if proven to work in younger children. We conducted an
Christoph T Behrendt et al.
Journal of medicinal chemistry, 54(19), 6796-6802 (2011-08-27)
Reverse hydroxamate-based inhibitors of IspC, a key enzyme of the non-mevalonate pathway of isoprenoid biosynthesis and a validated antimalarial target, were synthesized and biologically evaluated. The binding mode of one derivative in complex with EcIspC and a divalent metal ion
Karin Brücher et al.
Journal of medicinal chemistry, 55(14), 6566-6575 (2012-06-27)
Specific inhibition of enzymes of the non-mevalonate pathway is a promising strategy for the development of novel antiplasmodial drugs. α-Aryl-substituted β-oxa isosteres of fosmidomycin with a reverse orientation of the hydroxamic acid group were synthesized and evaluated for their inhibitory
Anne-Sophie Messiaen et al.
International journal of antimicrobial agents, 38(3), 261-264 (2011-07-05)
The Burkholderia cepacia complex (BCC) is a group of 17 closely related opportunistic pathogens that are able to infect the respiratory tract of cystic fibrosis patients. BCC bacteria are intrinsically resistant to many antibiotics and are therefore difficult to eradicate.
Matthew A DeSieno et al.
Chemical communications (Cambridge, England), 47(36), 10025-10027 (2011-08-11)
The Fe(II) and α-ketoglutarate-dependent hydroxylase FrbJ was previously demonstrated to utilize FR-900098 synthesizing a second phosphonate FR-33289. Here we assessed its ability to hydroxylate other possible substrates, generating a library of potential antimalarial compounds. Through a series of bioassays and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.