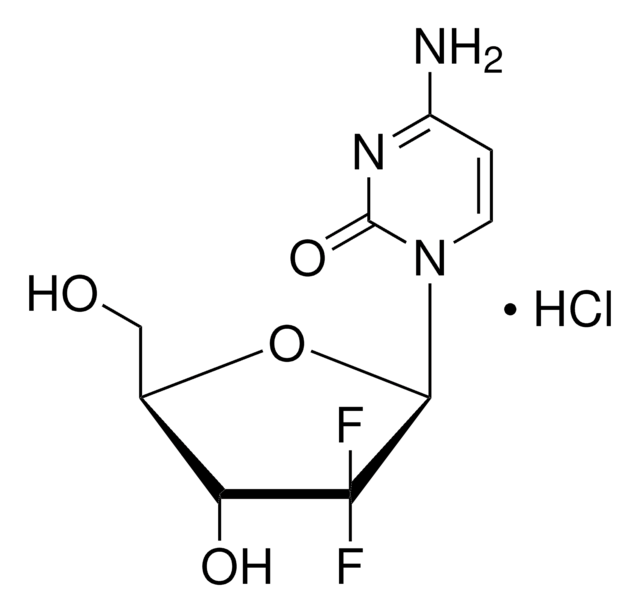
가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
생화학적/생리학적 작용
Doxifluridine is an antitumor agent efficient in tumors, cell lines or in fibroblasts transformed by H-ras or trk oncogenes. Possesses anticachectic activity which is independent of its antiproliferative activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Yujia Wen et al.
Pharmacogenetics and genomics, 21(8), 476-488 (2011-06-07)
To determine whether cellular apoptosis is a suitable phenotypic trait for pharmacogenomics studies by evaluating caspase 3/7-mediated activity in lymphoblastoid cell lines after treatment with six chemotherapeutic agents: 5'-deoxyfluorouridine, pemetrexed, cytarabine, paclitaxel, carboplatin, and cisplatin. Using monozygotic twin pair and
Gregory Lucien Bellot et al.
Journal of cancer research and clinical oncology, 138(3), 463-482 (2011-12-22)
Since primary tumor cells from patients have been used as a model for assessment of drug response for individual patients, this study aims to evaluate the reliability of such a model in colorectal cancer (CRC) in predicting the response of
Hitoshi Miyakoshi et al.
Journal of medicinal chemistry, 55(7), 2960-2969 (2012-03-13)
Recently, deoxyuridine triphosphatase (dUTPase) has emerged as a potential target for drug development as part of a new strategy of 5-fluorouracil-based combination chemotherapy. We have initiated a program to develop potent drug-like dUTPase inhibitors based on structure-activity relationship (SAR) studies
G J Peters et al.
International journal of cancer, 54(3), 450-455 (1993-05-28)
Malignant activation of oncogenes ras or trk is implicated in a number of solid tumors and leukemias. We determined the chemosensitivity profile of wild-type mouse NIH-3T3 fibroblasts, and that of NIH-3T3 lines transformed by the H-ras (S2-721) and trk (106-632)
Maria Ait-Tihyaty et al.
Breast cancer research and treatment, 133(1), 217-226 (2011-09-15)
Capecitabine (Xeloda) is a prodrug of 5-FU used in the clinical management of advanced breast cancer. It is metabolized first in the liver by carboxylesterases to generate 5'-deoxy-5-flurocytidine ribose (5'-DFCR), which is subsequently converted to 5'-deoxy-5-fluorouridine ribose (5'-DFUR) by cytidine
문서
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.