A7550
Achromopeptidase from Achromobacter lyticus
lyophilized powder, Protein ~5 % by biuret, 300-600 units/mg solid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
Recommended Products
form
lyophilized powder
specific activity
300-600 units/mg solid
composition
Protein, ~5% biuret
foreign activity
Collagenase, present
storage temp.
−20°C
Biochem/physiol Actions
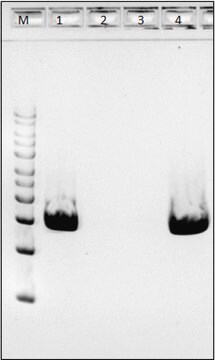
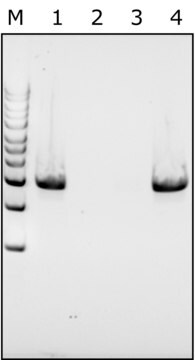
Achromopeptidase is a lysyl endopeptidase with a MW of ~27 kDa. It is useful for lysis of Gram-positive bacteria that are resistant to lysozyme.
pH Optimum for activity: pH 8.5 - 9
Approximately 500-1,500 un/ml achromopetidase can be used to lyse cells at a density of OD600=0.6 over 2 hours at 37 °C.
pH Optimum for activity: pH 8.5 - 9
Approximately 500-1,500 un/ml achromopetidase can be used to lyse cells at a density of OD600=0.6 over 2 hours at 37 °C.
Unit Definition
One unit will produce a change in A600 of 0.001 per minute per mL at pH 8.0 at 37 °C using a suspension of Micrococcus lysodeikticus as substrate (1 cm light path).
Physical form
Crude powder containing salts and medium components
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Beatrice Quevedo et al.
BMC microbiology, 11, 14-14 (2011-01-21)
The purpose of this study was to design and evaluate fluorescent in situ hybridization (FISH) probes for the single-cell detection and enumeration of lactic acid bacteria, in particular organisms belonging to the major phylogenetic groups and species of oral lactobacilli
Niamh Toomey et al.
Applied and environmental microbiology, 75(10), 3146-3152 (2009-03-10)
Three wild-type dairy isolates of lactic acid bacteria (LAB) and one Lactococcus lactis control strain were analyzed for their ability to transfer antibiotic resistance determinants (plasmid or transposon located) to two LAB recipients using both in vitro methods and in
Parul A Patel et al.
Journal of clinical microbiology, 49(6), 2266-2268 (2011-04-22)
We evaluated the BD GeneOhm MRSA achromopeptidase (ACP) assay, which incorporates a new specimen preparation approach. A total of 1,216 leftover nasal samples were tested; using culture as the gold standard, the sensitivity and specificity were 92% and 94.6%, respectively.
Toshio Tomita et al.
The Journal of biological chemistry, 279(52), 54161-54172 (2004-10-19)
Flammutoxin (FTX), a 31-kDa pore-forming cytolysin from Flammulina velutipes, is specifically expressed during the fruiting body formation. We cloned and expressed the cDNA encoding a 272-residue protein with an identical N-terminal sequence with that of FTX but failed to obtain
High yield preparation of genomic DNA from Streptomyces.
Jasmina Nikodinovic et al.
BioTechniques, 35(5), 932-934 (2003-11-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service