557330
Rolipram
A cell-permeable, selective inhibitor of cAMP-specific phosphodiesterase (PDE IV; IC₅₀ = 800 nM).
Synonym(s):
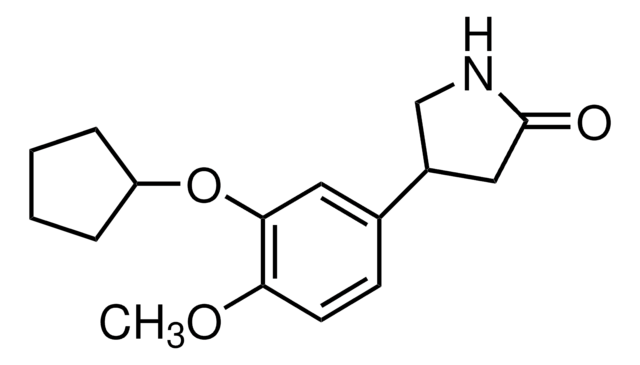
Rolipram, 4-[3-(Cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C16H21NO3
CAS Number:
Molecular Weight:
275.34
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
description
Merck USA index - 14, 8251
Assay
≥98% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
color
white
solubility
DMSO: soluble
shipped in
ambient
storage temp.
2-8°C
General description
A cell-permeable, selective inhibitor of cAMP-specific phosphodiesterase (PDE IV; IC50 = 800 nM). A rolipram-insensitive PDE IV subtype is also known to exist. Also inhibits NF-κB and NFAT activation in Jurkat and primary T cells.
Cell permeable, selective competitive inhibitor of cAMP-specific phosphodiesterase IV (IC50 = 800 nM). Rolipram does not inhibit PDE I or PDE II, even at concentrations as high as 100 µM. It is only a weak inhibitor of PDE III (IC50 = 100 µM). A rolipram-insensitive phosphodiesterase IV subtype is also known to exist.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
PDE IV
PDE IV
Product does not compete with ATP.
Reversible: no
Target IC50: 800 nM against cAMP-specific phosphodiesterase (PDE IV)
Warning
Toxicity: Irritant (B)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). DMSO stock solutions are stable for up to 6 months at -20°C.
Other Notes
Navarro, J., et al. 1998. J. Virol. 72, 4712.
Truong, V.H. and Muller, T. 1994. FEBS Lett.353, 113.
Underwood, D.C., et al. 1994. J. Pharmacol. Exp. Ther. 270, 250.
Moore, J.B., Jr., et al. 1991. Biochem. Pharmacol.42, 679.
Reeves, M.L., et al. 1987. Biochem. J. 241, 535.
Schneider, H.H., et al. 1986. Eur. J. Pharmacol.127, 105.
Truong, V.H. and Muller, T. 1994. FEBS Lett.353, 113.
Underwood, D.C., et al. 1994. J. Pharmacol. Exp. Ther. 270, 250.
Moore, J.B., Jr., et al. 1991. Biochem. Pharmacol.42, 679.
Reeves, M.L., et al. 1987. Biochem. J. 241, 535.
Schneider, H.H., et al. 1986. Eur. J. Pharmacol.127, 105.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Line M Grønning et al.
FEBS letters, 580(17), 4126-4130 (2006-07-11)
Overexpression of forkhead transcription factor FOXC2 in white adipose tissue (WAT) leads to a lean phenotype resistant to diet-induced obesity. This is due, in part, to enhanced catecholamine-induced cAMP-PKA signaling in FOXC2 transgenic mice. Here we show that rolipram treatment
H Ji
Methods in molecular medicine, 51, 363-377 (2001-01-01)
Angiotensin type-1 receptors (AT(1) receptors) mediate various physiological actions of angiotensin (Ang II) via multiple-signal transduction pathways (1). In addition to the phospholipase C pathway and dihydropyridine-sensitive voltage-dependent calcium channels, AT(1) receptors can couple to inhibition of adenylate cyclase via
Sriram Hemachandran et al.
Frontiers in cellular neuroscience, 18, 1363219-1363219 (2024-05-02)
Cochlear afferent synapses connecting inner hair cells to spiral ganglion neurons are susceptible to excitotoxic trauma on exposure to loud sound, resulting in a noise-induced cochlear synaptopathy (NICS). Here we assessed the ability of cyclic AMP-dependent protein kinase (PKA) signaling
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service