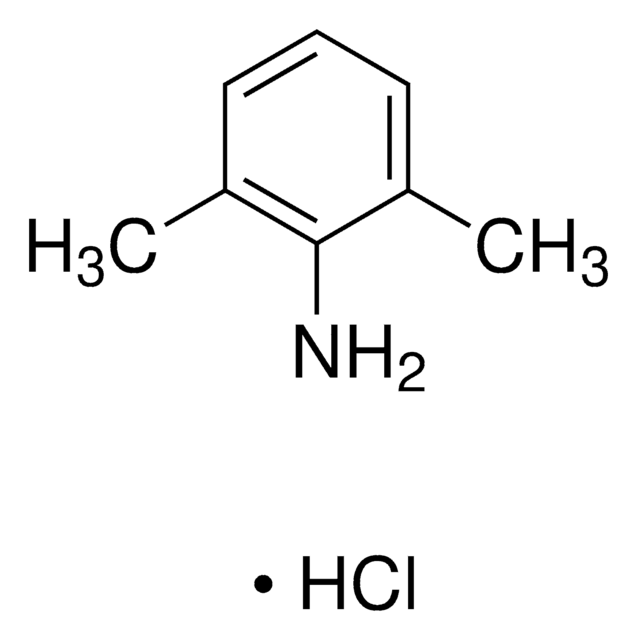
PHR1257
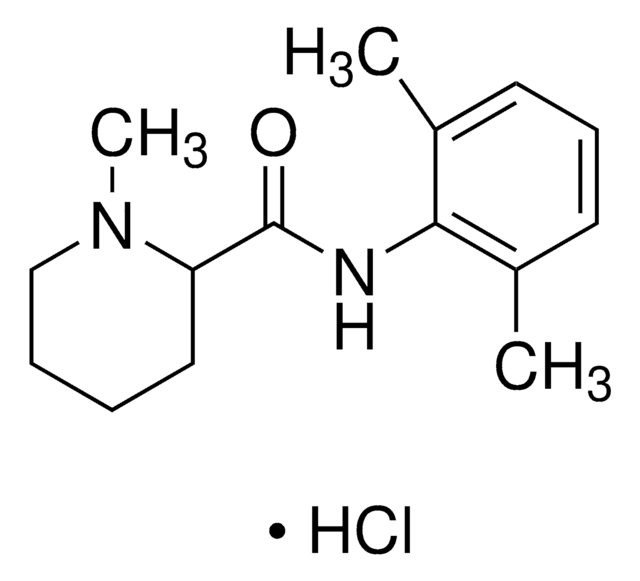
Lidocaine hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Lidocaine hydrochloride monohydrate, 2-Diethylamino-N-(2,6-dimethylphenyl)acetamide hydrochloride monohydrate, Lignocaine hydrochloride monohydrate, Xylocaine hydrochloride monohydrate
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to BP 214
traceable to Ph. Eur. L0600000
traceable to USP 1366013
API family
lidocaine
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
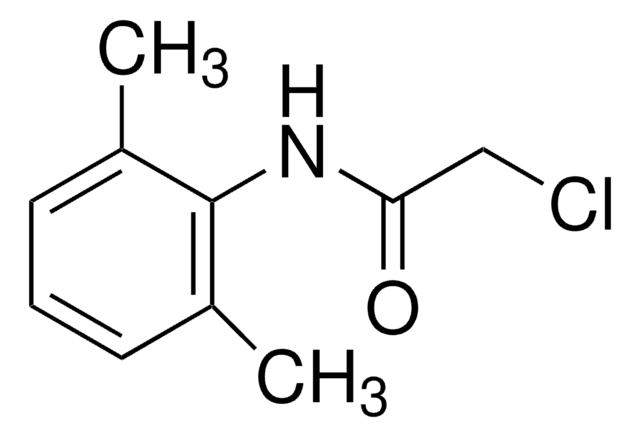
Cl[H].[H]O[H].CCN(CC)CC(=O)Nc1c(C)cccc1C
InChI
1S/C14H22N2O.ClH.H2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H;1H2
InChI key
YECIFGHRMFEPJK-UHFFFAOYSA-N
Gene Information
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Lidocaine hydrochloride is an amide type local anaesthetic that belongs to the class of 1b antiarrhythmics. It is used for regional nerve blocks and infiltrative administration of anaesthesia. It prevents the entry of sodium ions into nerve endings, at the site of pain, and disrupts the electrical signal from reaching the brain.
Application
- Sensitive determination of lidocaine hydrochloride and cetylpyridinium chloride in their binary mixtures in different pharmaceutical formulations by three spectrophotometric-based methods
- Simultaneous analysis of aminoacridine hydrochloride and lidocaine hydrochloride in bulk powder and pharmaceutical formulation by high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC)-densitometric methods
- Quantification of ceftriaxone sodium and lidocaine HCl in human plasma samples by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS)
- Quantitative analysis of lidocaine hydrochloride and miramistin in a wound healing gel sample by high-performance liquid chromatography (HPLC) combined with UV detection
- Development of a method based on liquid–liquid extraction of nifedipine and lidocaine hydrochloride from human plasma samples for their subsequent analysis by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)
- Dispersive liquid-liquid microextraction (DLLME) followed by attenuated total reflectance-Fourier transform infrared measurement of dry films for the determination of lidocaine hydrochloride in human urine sample
Biochem/physiol Actions
Analysis Note
Footnote
Recommended products
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service