15450
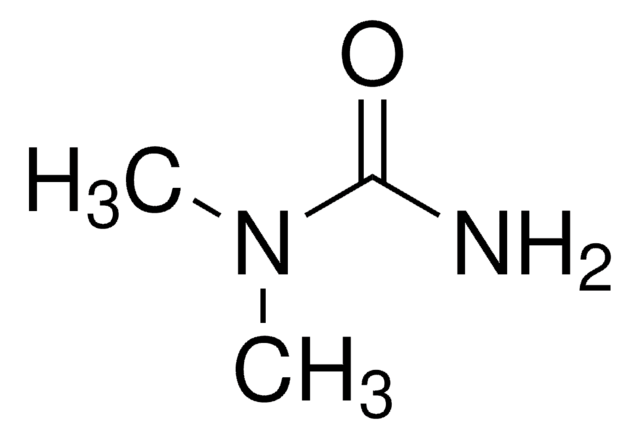
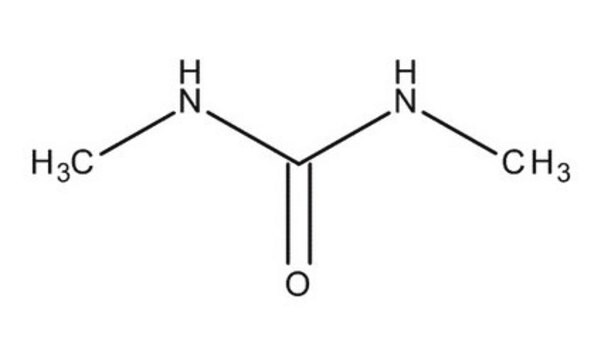
N,N′-Dimethylurea
(sym.), ≥99% (from N)
Synonym(s):
DMU, 1,3-Dimethylurea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3NH)2CO
CAS Number:
Molecular Weight:
88.11
Beilstein:
1740672
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99% (from N)
form
solid
reaction suitability
reagent type: ligand
reaction type: C-H Activation
bp
268-270 °C (lit.)
mp
101-104 °C (lit.)
103-107 °C
SMILES string
CNC(=O)NC
InChI
1S/C3H8N2O/c1-4-3(6)5-2/h1-2H3,(H2,4,5,6)
InChI key
MGJKQDOBUOMPEZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N′-Dimethylurea can be used:
- As a starting material to synthesize N,N′-dimethyl-6-amino uracil.
- In combination with β-cyclodextrin derivatives, to form low melting mixtures (LMMs), which can be used as solvents for hydroformylation and Tsuji-Trost reactions.
- To synthesize N,N′-disubstituted-4-aryl-3,4-dihydropyrimidinones via Biginelli condensation under solvent-free conditions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
314.6 °F - closed cup
Flash Point(C)
157 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of 1, 3-Dimethyl-5, 6-diaminouracil.
Blicke F F and Godt Jr H C
Journal of the American Chemical Society, 76(10), 2798-2800 (1954)
Low melting mixtures based on β-cyclodextrin derivatives and N, N′-dimethylurea as solvents for sustainable catalytic processes.
Jerome F, et al.
Green Chemistry, 16(8), 3876-3880 (2014)
Hyun-Woong Cho et al.
PloS one, 14(6), e0217745-e0217745 (2019-06-21)
The aim of this study was to investigate the short-term efficacy and safety of Poly-gamma-glutamic acid (γ-PGA) and the immunologic changes in patients with CIN 1. Participants were randomly assigned to one of two groups and orally treated with placebo
Dowex-promoted general synthesis of N, N'-disubstituted-4-aryl-3, 4-dihydropyrimidinones using a solvent-free Biginelli condensation protocol.
Singh K, et al.
Tetrahedron Letters, 47(25), 4205-4207 (2006)
Anna Ronowicz et al.
Analytical biochemistry, 426(2), 91-93 (2012-04-17)
Reuse of materials in DNA hybridization-based methods has been known since the advent of Southern membranes. Array-based comparative genomic hybridization is essentially Southern hybridization with multiple probes immobilized on a solid surface. We show that comparative genomic hybridization microarrays fabricated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service