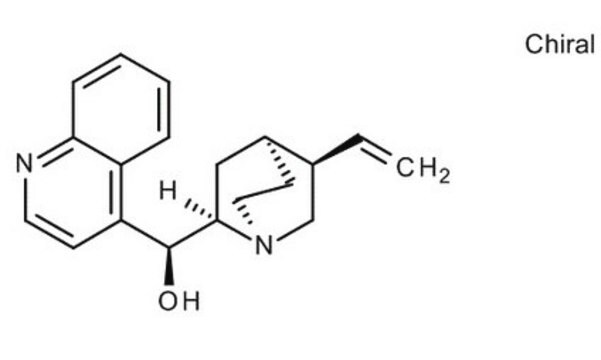
22600
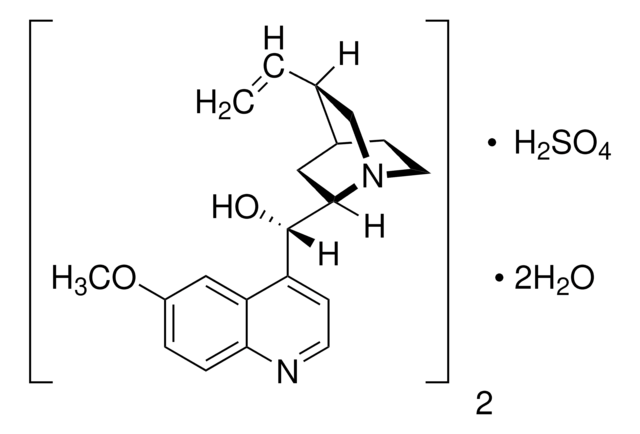
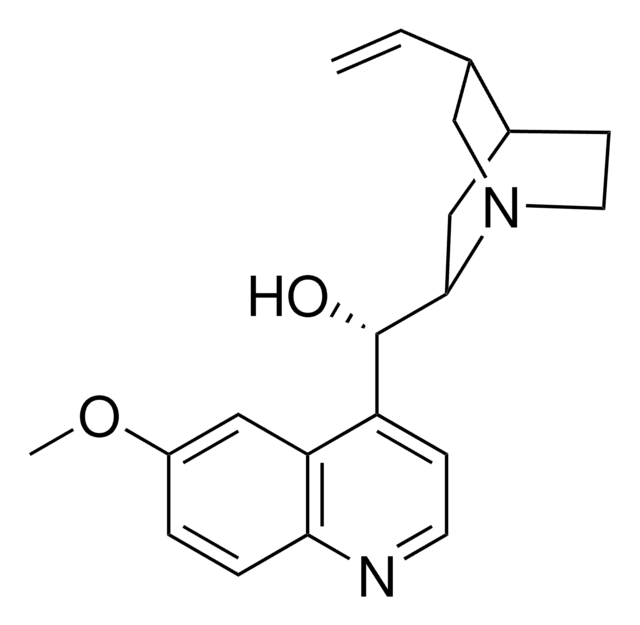
Quinidine
crystallized, ≥98.0% (dried material, NT)
Synonym(s):
β-Quinidine, (+)-Quinidine, (9S)-6′-Methoxycinchonan-9-ol, Chinidin
About This Item
Recommended Products
Quality Level
Assay
≥98.0% (dried material, NT)
form
solid
optical activity
[α]20/D +265±5°, c = 0.8% in ethanol (dry matter)
quality
crystallized
impurities
≤2% water
~10% hydroquinidine (HPLC)
mp
168-172 °C (lit.)
functional group
hydroxyl
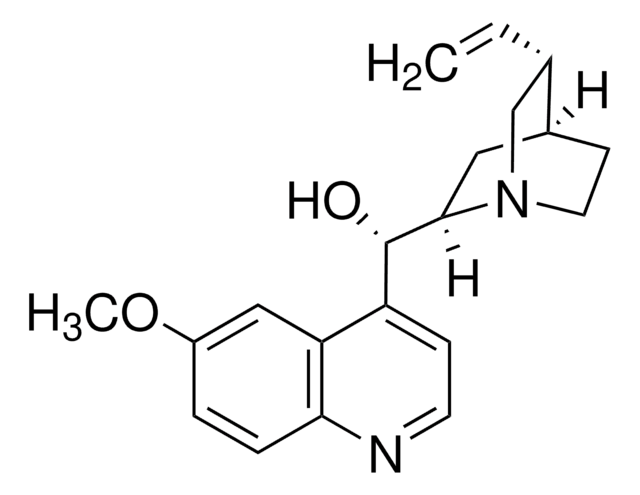
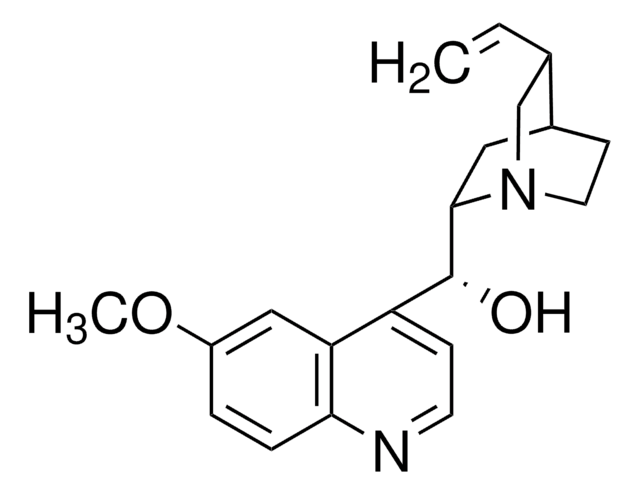
SMILES string
COc1ccc2nccc([C@H](O)C3CC4CCN3C[C@@H]4C=C)c2c1
InChI
1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1
InChI key
LOUPRKONTZGTKE-LHHVKLHASA-N
Gene Information
human ... ABCB1(5243) , CYP2D6(1565) , CYP3A4(1576) , KCNH1(3756) , SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
mouse ... Abcb1a(18671) , Abcb1b(18669)
rat ... Cyp2d1(266684) , Cyp2d2(25053) , Cyp2d3(24303) , Cyp2d4v1(171522)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
LC/MS/MS Analysis of Interacting Cardiac Drugs Digoxin, Quinidine, Amiodarone and Verapamil on Titan™ C18
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service