37760
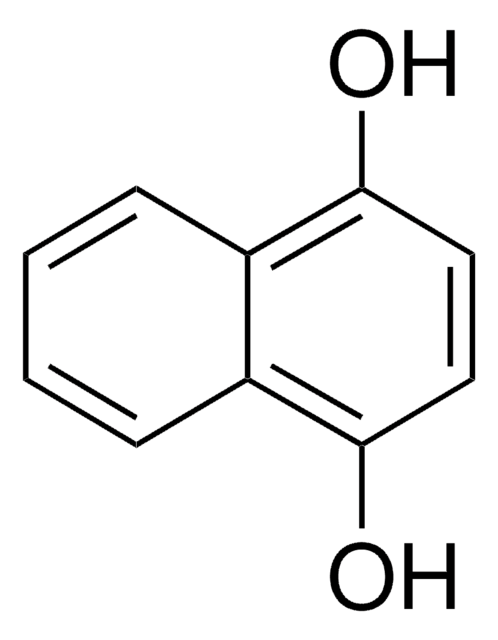
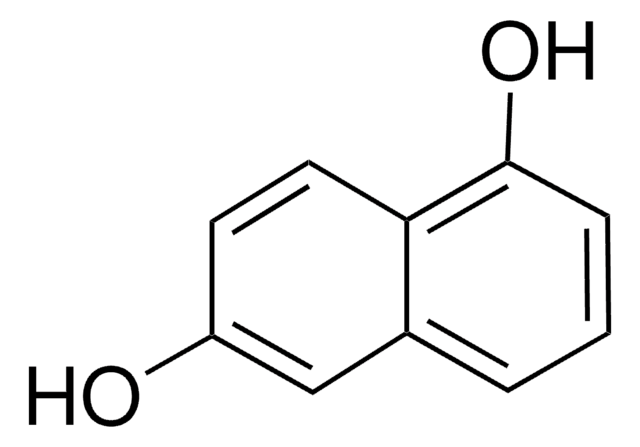
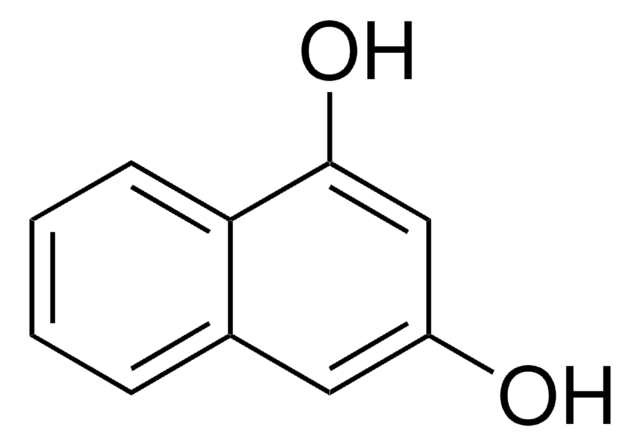
2,3-Dihydroxynaphthalene
≥98.0% (HPLC)
Synonym(s):
2,3-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
Beilstein:
742375
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
sublimation residue
≤1%
mp
161-165 °C (lit.)
162-164 °C
SMILES string
Oc1cc2ccccc2cc1O
InChI
1S/C10H8O2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6,11-12H
InChI key
JRNGUTKWMSBIBF-UHFFFAOYSA-N
Gene Information
human ... BAD(572)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,3-Dihydroxynaphthalene is a polyhydroxy phenol. It is an aromatic dihydroxy compound having hydroxyl groups at ortho positions. Its reaction with molybdenum(VI) complexes has been reported. The asymmetric oxidative coupling polymerization of 2,3-dihydroxynaphthalene using the Cu(I)-bisoxazoline complex as catalyst has been reported to afford poly(2,3-dihydroxy-1,4-naphthylene), having a continuous 1,1′-bi-2-naphthol main chain structure. The nitrodisplacement reaction between nitrophthalodinitriles and 2,3-dihydroxynaphthalene has been investigated.
Application
2,3-Dihydroxynaphthalene may be used in the following studies:
- Construction of dinaphtho[2,1-b;2′,3′-d]furan-6-ol, via dehydration reaction in the presence of strong acid.
- As fused ring catecholate type ligand for the surface modification of nanocrystalline TiO2 particles.
- As adsorptive and competing ligand during the chemical speciation of iron in seawater by cathodic stripping voltammetry.
- Synthesis of cyclotriphosphazene derivatives, used as non-halogen flame retardants
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
347.0 °F
Flash Point(C)
175 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Copper (I)-catalyzed asymmetric oxidative coupling polymerization of 2, 3-dihydroxynaphthalene using bisoxazoline ligands.
Habaue S, et al.
Macromolecules, 36(8), 2604-2608 (2003)
Application of cyclophosphazene derivatives as flame retardants for ABS.
Shin YJ, et al.
Journal of Industrial and Engineering Chemistry (Amsterdam, Netherlands), 16(3), 364-367 (2010)
Tatjana D Savić et al.
Nanoscale, 4(5), 1612-1619 (2012-02-09)
Surface modification of nanocrystalline TiO(2) particles (45 Å) with catecholate-type ligands consisting of an extended aromatic ring system, i.e., 2,3-dihydroxynaphthalene and anthrarobin, was found to alter the optical properties of the nanoparticles in a similar way to modification with catechol.
Synthesis of bis (ether anhydride) s for poly (ether imide) s having 1, 2-linked units by nitrodisplacement with catechol derivatives.
Eastmond GC and Paprotny J.
Macromolecules, 28(7), 2140-2146 (1995)
Chemical speciation of iron in seawater by cathodic stripping voltammetry with dihydroxynaphthalene.
Constant M G van den Berg
Analytical chemistry, 78(1), 156-163 (2005-12-31)
The chemical speciation of iron in seawater is determined by cathodic stripping voltammetry using 2,3-dihydroxynaphthalene (DHN) as adsorptive and competing ligand. The optimized conditions include a DHN concentration of 0.5-1 microM, seawater at its original pH of 8, and equilibration
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service