389579
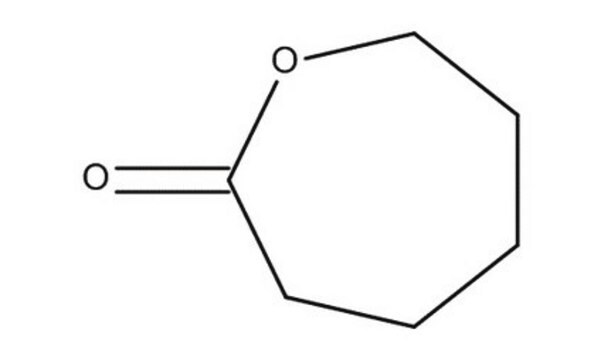
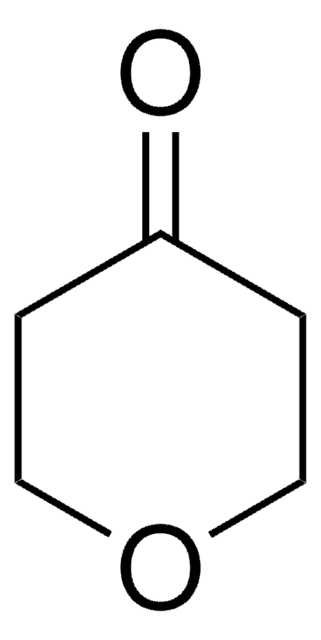
δ-Valerolactone
technical grade
Synonym(s):
delta-Valerolactone, Tetrahydro-2H-2-pyranone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H8O2
CAS Number:
Molecular Weight:
100.12
Beilstein:
106436
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
liquid
impurities
<25% polymer
refractive index
n20/D 1.457 (lit.)
bp
226-229 °C (lit.)
58-60 °C/0.5 mmHg (lit.)
mp
−13-−12 °C (lit.)
density
1.079 g/mL at 25 °C (lit.)
functional group
ester
storage temp.
−20°C
SMILES string
O=C1CCCCO1
InChI
1S/C5H8O2/c6-5-3-1-2-4-7-5/h1-4H2
InChI key
OZJPLYNZGCXSJM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
δ-Valerolactone (tetrahydro-2H-2-pyranone or δ VL) can be used as a monomer unit in the synthesis of poly(δ-valerolactone)s poly(conjugated ester)s via ring-opening polymerization.
It can also be used as a starting material in the synthesis of (+)-guadinomic acid , sodium δ-hydroxyvalerate , methyl δ-hydroxyvalerate , and 5-hydroxyvaleraldehyde.
It can also be used as a starting material in the synthesis of (+)-guadinomic acid , sodium δ-hydroxyvalerate , methyl δ-hydroxyvalerate , and 5-hydroxyvaleraldehyde.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K N Houk et al.
The Journal of organic chemistry, 73(7), 2674-2678 (2008-03-08)
gamma-Butyrolactone, unlike delta-valerolactone, does not polymerize despite a strain energy of approximately 8 kcal mol-1 which could be relieved by opening the s-cis lactone ester bond to an s-trans ester bond in the polymer. To explain this anomaly, we have
Organolanthanide-initiated living polymerizations of ε -caprolactone, δ-valerolactone, and β-propiolactone
Yamashita M, et al.
Macromolecules, 29(5), 1798-1806 (1996)
Zuwei Ma et al.
Biomacromolecules, 12(9), 3265-3274 (2011-07-16)
Biodegradable polyurethane urea (PUU) elastomers are ideal candidates for fabricating tissue engineering scaffolds with mechanical properties akin to strong and resilient soft tissues. PUU with a crystalline poly(ε-caprolactone) (PCL) macrodiol soft segment (SS) showed good elasticity and resilience at small
Ryo Shintani et al.
Organic letters, 14(9), 2410-2413 (2012-04-26)
A palladium-catalyzed decarboxylative cyclopropanation of γ-methylidene-δ-valerolactones with aromatic aldehydes has been developed to give 4-oxaspiro[2.4]heptanes with high selectivity. The site of nucleophilic attack to a π-allylpalladium intermediate has been controlled with a sterically demanding phosphine ligand. The course of the
Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone
Makiguchi K, et al.
Macromolecules, 44(7), 1999-2005 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service