V209
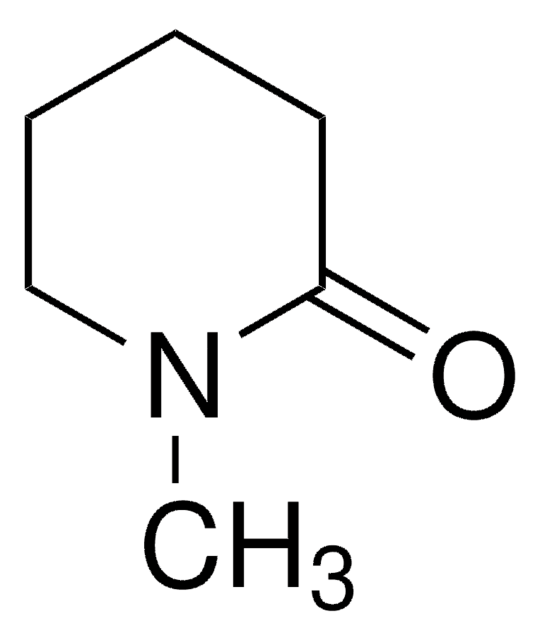
δ-Valerolactam
98%
Synonym(s):
delta-Valerolactam, 2-Piperidone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO
CAS Number:
Molecular Weight:
99.13
Beilstein:
106434
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
256 °C (lit.)
81-82 °C/0.1 mmHg (lit.)
mp
38-40 °C (lit.)
SMILES string
O=C1CCCCN1
InChI
1S/C5H9NO/c7-5-3-1-2-4-6-5/h1-4H2,(H,6,7)
InChI key
XUWHAWMETYGRKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Gordon et al.
Farmaco (Societa chimica italiana : 1989), 52(10), 603-608 (1998-05-15)
The synthesis of a series of 2-amino-4-hydroxy-delta-valerolactam derivatives is described (compounds 4 to 10). These compounds showed a high anthelmintic in vitro activity against the Nippostrongylus brasiliensis model.
Hiroshi Tsuchikawa et al.
Organic letters, 14(9), 2326-2329 (2012-04-26)
Chiral 2-piperidinone compounds with various C-6 substituents were successfully synthesized via a Pd-catalyzed asymmetric 6-endo cyclization of dienamides, which were evidently activated by both N-p-toluenesulfonyl and C-3 ester substituents.
Zheng Hu et al.
Chemosphere, 268, 128834-128834 (2020-11-11)
A magnetic Ag3PO4/rGO/CoFe2O4 ternary catalyst was firstly prepared and used for removing levofloxacin (LVF) from different water matrices via simultaneous adsorption and photocatalysis. Compared with Ag3PO4 and Ag3PO4/CoFe2O4, Ag3PO4/rGO/CoFe2O4 shows a superior adsorption-photocatalysis performance for LVF elimination since rGO component
Asymmetric synthesis of gamma-keto-delta-lactam derivatives: application to the synthesis of a conformationally constrained surrogate of Ala-Ser dipeptide.
S D Koulocheri et al.
The Journal of organic chemistry, 66(23), 7915-7918 (2001-11-10)
Moitrayee Mukherjee et al.
The journal of physical chemistry. A, 116(40), 9888-9896 (2012-09-19)
A comparative analysis for relative stability between normal and tautomeric forms in the excited electronic states of 7-azaindole···δ-valerolactam 1:1 complex and 7-azaindole homodimer has been presented. The tautomeric configuration of the complex is estimated to be ~6 kcal/mol more stable
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service