909327
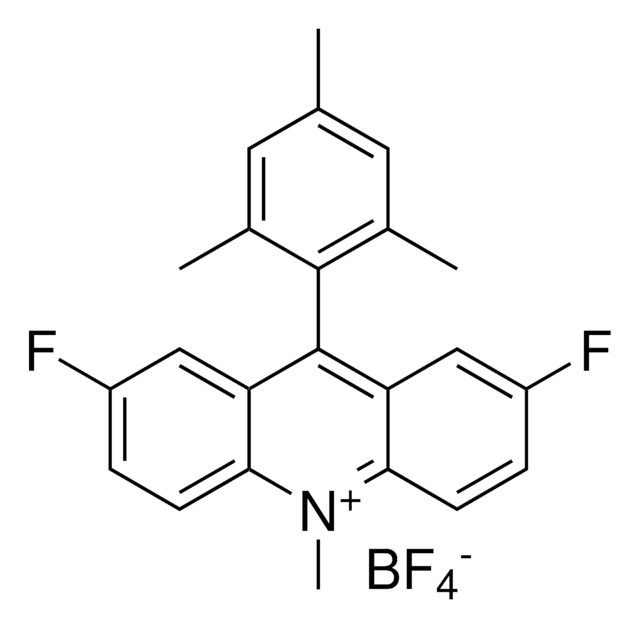
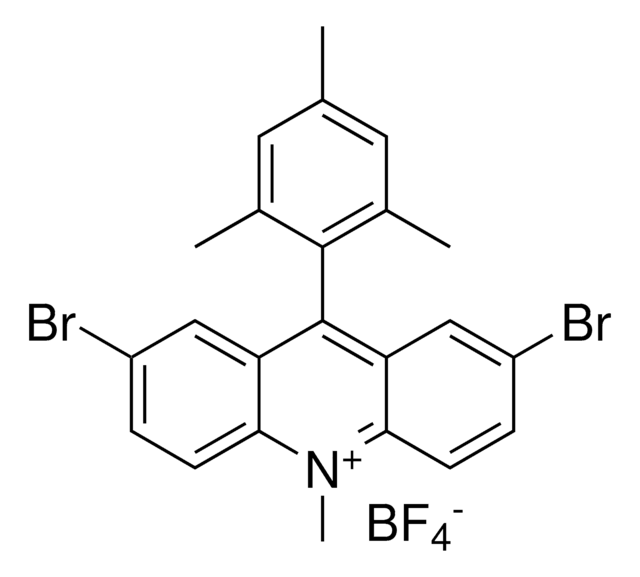
2,7-Dimethoxy-9-mesityl-10-methylacridinium tetrafluoroborate
About This Item
Recommended Products
form
powder
reaction suitability
reaction type: Photocatalysis
reagent type: catalyst
SMILES string
[F-][B+3]([F-])([F-])[F-].O(C=1C=CC2=C(C1)C(=C3C=C(OC)C=CC3=[N+]2C)C4=C(C=C(C=C4C)C)C)C
InChI
InChI=1S/C25H26NO2.BF4/c1-15-11-16(2)24(17(3)12-15)25-20-13-18(27-5)7-9-22(20)26(4)23-10-8-19(28-6)14-21(23)25;2-1(3,4)5/h7-14H,1-6H3;/q+1;-1
InChI key
NEMWBWBWRDTDNA-UHFFFAOYSA-N
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Nicewicz lab is focused on the discovery of new and powerful reaction methodologies that proceed via the intermediacy of highly reactive cation radical species. Included in these transformations are anti-Markovnikov selective additions of amines, alcohols, carboxylic acids, amides and mineral acids to alkenes.
Photoredox catalysis utilizes photocatalysts in the presence of visible light to form an array of synthetic transformations including, but not limited to, cross-coupling, C-H functionalization, alkene and arene functionalization, and trifluoromethylation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service