H56800
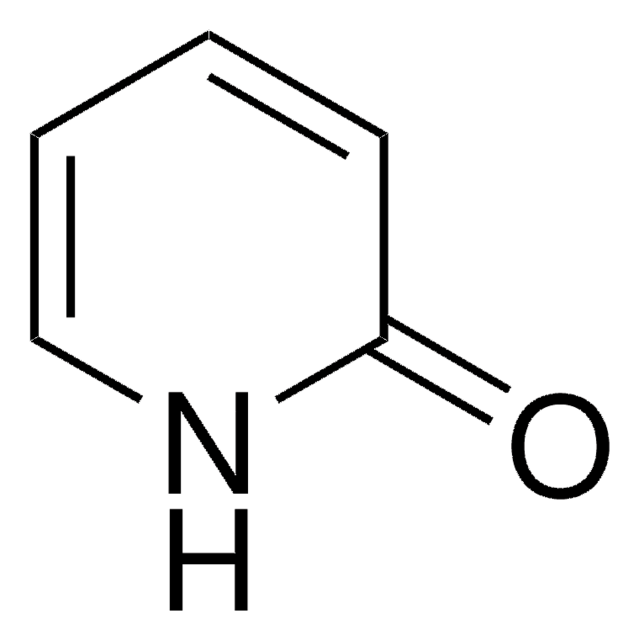
2-Hydroxypyridine
97%
Synonym(s):
2(1H)-Pyridone, 2-Pyridinol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105757
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
280-281 °C (lit.)
mp
105-107 °C (lit.)
SMILES string
Oc1ccccn1
InChI
1S/C5H5NO/c7-5-3-1-2-4-6-5/h1-4H,(H,6,7)
InChI key
UBQKCCHYAOITMY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Catalyst for generating β-oxopropyl carbonates from cyclic carbonates and alcohols and in the aminolysis of a polyglutamate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the Chemical Society. Perkin Transactions 1, 1749-1749 (1993)
Ali A Abdul-Sater et al.
Journal of immunology (Baltimore, Md. : 1950), 195(1), 210-216 (2015-05-29)
IFNs, which transduce pivotal signals through Stat1 and Stat2, effectively suppress the replication of Legionella pneumophila in primary murine macrophages. Although the ability of IFN-γ to impede L. pneumophila growth is fully dependent on Stat1, IFN-αβ unexpectedly suppresses L. pneumophila
Polymer, 35, 2443-2443 (1994)
Susan Blaser et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 12(10), 1841-1850 (2011-05-25)
A combined spectroscopic and ab initio theoretical study of the doubly hydrogen-bonded complex of 2-pyridone (2PY) with NH(3) has been performed. The S(1)←S(0) spectrum extends up to ≈1200 cm(-1) above the 0(0) (0) band, close to twice the range observed
Mettu Ravinder et al.
Bioorganic & medicinal chemistry letters, 22(18), 6010-6015 (2012-08-18)
Twenty-six 2-pyridone derivatives (8a-8z), which are structurally analogous to amrinone and milrinone two important cardiotonic drugs, are synthesized and characterized. The synthesis of 2-pyridone derivatives involves addition, followed by cyclization between Baylis-Hillman acetates (7a-7k) and enamino esters or nitriles (3a-3e).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service