553210
Rapamycin
≥95% (HPLC), powder
Synonym(s):
Rapamycin, mTOR Inhibitor I
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
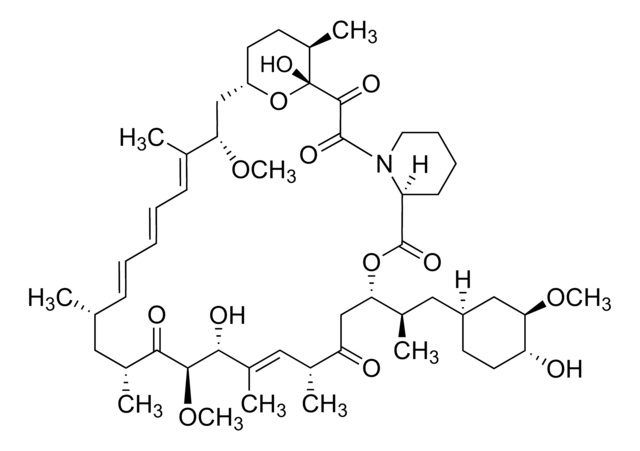
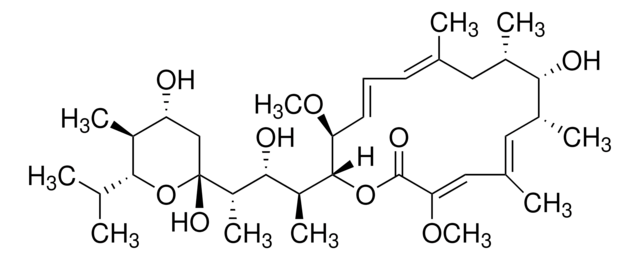
C51H79NO13
CAS Number:
Molecular Weight:
914.17
MDL number:
UNSPSC Code:
41116107
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
powder
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
color
clear
solubility
DMSO: 200 mg/mL
ethanol: 50 mg/mL
shipped in
ambient
storage temp.
−20°C
General description
Anti-fungal and immunosuppressant. Selectively inhibits the mammalian target of rapamycin (mTOR) and blocks the subsequent activation of p70 S6 kinase (IC50 = 50 pM). Specifically inhibits mTORC1, but mTORC2 that phosphorylates Akt at Ser473 appears to be insensitive to rapamycin. Prevents the translational activation of IGF-II. Also prevents resting T-cells from entering the cell cycle, but does not directly arrest cell cycle progression. Shown to inhibit later signaling events, such as p110Rb phosphorylation, p34cdc2 kinase activation, and cyclin A synthesis. Exhibits strong binding to FK-506 binding proteins. Also reported to induce apoptosis in a murine B-cell line, to inhibit lymphokine-induced cell proliferation at the G1 phase, and to irreversibly arrest Saccharomyces cerevisiae cells in the G1 phase. A 5 mM (500 µg/109 µl) solution of Rapamycin (Cat. No. 553211) in DMSO and a 10 mM (1 mg/109 µl) solution of Rapamycin (Cat. No. 553212) in EtOH is also available.
Selectively inhibits the mammalian target of rapamycin (mTOR) and blocks the subsequent activation of p70 S6 kinase (IC50 = 50 pM). Specifically inhibits mTORC1, but mTORC2 that phosphorylates Akt at Ser473 appears to be insensitive to rapamycin. Prevents the translational activation of IGF-II. Also prevents resting T-cells from entering the cell cycle, but does not directly arrest cell cycle progression. Shown to inhibit later signaling events, such as p110Rb phosphorylation, p34cdc2 kinase activation, and cyclin A synthesis. Exhibits strong binding to FK-506 binding proteins. Also reported to induce apoptosis in a murine B-cell line, to inhibit lymphokine-induced cell proliferation at the G1 phase, and to irreversibly arrest Saccharomyces cerevisiae cells in the G1 phase.
Biochem/physiol Actions
Cell permeable: no
Primary Target
Mammalian target of rapamycin (mTOR)
Mammalian target of rapamycin (mTOR)
Product does not compete with ATP.
Reversible: no
Target IC50: 50 pM against p70 S6 kinase
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Other Notes
Chen, T., et al. 2011. Aging Cell.10, 908.
Powell, D.J., et al. 2003. Mol. cell Biol.23, 7794.
Braun, W., et al. 1995. FASEB J.9, 63.
Nielsen, F.C., et al. 1995. Nature 377, 358.
Aagaard-Tillery, K.M. and Jelinek, D.F. 1994. Cell. Immunol. 156, 493.
Gottschalk, A.R., et al. 1994. Proc. Natl. Acad. Sci. USA91, 7350.
Morice, W.G., et al. 1993. J. Biol. Chem.268, 3734.
Terada, N., et al. 1993. J. Biol. Chem.268, 12062.
Kuo, J., et al. 1992. Nature 358, 70.
Price, D.J., et al. 1992. Science257, 973.
Heitman, J., et al. 1991. Science253, 905.
Kay, J.E., et al. 1991. Immunology72, 544.
Schreiber, S.L. 1991. Science251, 283.
Bierer, B.E., et al. 1990. Proc. Natl. Acad. Sci. USA87, 9231.
Dumont, F.J., et al. 1990. J. Immunol.144, 251.
Powell, D.J., et al. 2003. Mol. cell Biol.23, 7794.
Braun, W., et al. 1995. FASEB J.9, 63.
Nielsen, F.C., et al. 1995. Nature 377, 358.
Aagaard-Tillery, K.M. and Jelinek, D.F. 1994. Cell. Immunol. 156, 493.
Gottschalk, A.R., et al. 1994. Proc. Natl. Acad. Sci. USA91, 7350.
Morice, W.G., et al. 1993. J. Biol. Chem.268, 3734.
Terada, N., et al. 1993. J. Biol. Chem.268, 12062.
Kuo, J., et al. 1992. Nature 358, 70.
Price, D.J., et al. 1992. Science257, 973.
Heitman, J., et al. 1991. Science253, 905.
Kay, J.E., et al. 1991. Immunology72, 544.
Schreiber, S.L. 1991. Science251, 283.
Bierer, B.E., et al. 1990. Proc. Natl. Acad. Sci. USA87, 9231.
Dumont, F.J., et al. 1990. J. Immunol.144, 251.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Erika E Kuchen et al.
eLife, 9 (2020-01-24)
Cell heterogeneity may be caused by stochastic or deterministic effects. The inheritance of regulators through cell division is a key deterministic force, but identifying inheritance effects in a systematic manner has been challenging. Here, we measure and analyze cell cycles
Zhigang Zhang et al.
Aging, 12(18), 17786-17799 (2020-09-23)
Rapamycin delays multiple age-related conditions and extends lifespan in organisms ranging from yeast to mice. However, the mechanisms by which rapamycin influences longevity are incompletely understood. The objective of this study was to investigate the effect of rapamycin on NAD+/NADH
Isis M Ornelas et al.
Glia, 68(6), 1274-1290 (2020-01-07)
Oligodendrocyte precursor cells (OPCs) differentiate and mature into oligodendrocytes, which produce myelin in the central nervous system. Prior studies have shown that the mechanistic target of rapamycin (mTOR) is necessary for proper myelination of the mouse spinal cord and that
Jaclyn E Welles et al.
American journal of physiology. Endocrinology and metabolism (2020-05-19)
Fibroblast growth factor 21 (FGF21) is a peptide hormone that acts to enhance insulin sensitivity and reverse many of the metabolic defects associated with consumption of a high-fat diet. Recent studies show that the liver is the primary source of
Christopher A Jackson et al.
eLife, 9 (2020-01-28)
Understanding how gene expression programs are controlled requires identifying regulatory relationships between transcription factors and target genes. Gene regulatory networks are typically constructed from gene expression data acquired following genetic perturbation or environmental stimulus. Single-cell RNA sequencing (scRNAseq) captures the
Articles
AldeRed™ 588-A detects ALDH activity in cancer stem cells, aiding in cancer research.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service