R2625
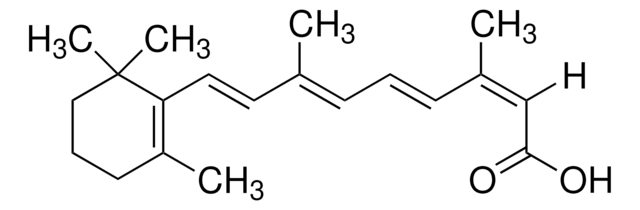
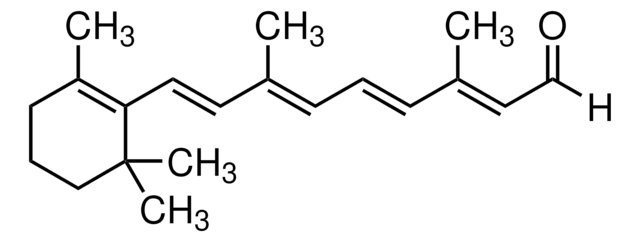
Retinoic acid
≥98% (HPLC), powder, RAR and RXR ligand
Synonym(s):
ATRA, Tretinoin, Vitamin A acid, all-trans-Retinoic acid
Select a Size
Select a Size
About This Item
Recommended Products
Product Name
Retinoic acid, ≥98% (HPLC), powder
biological source
synthetic (organic)
Quality Level
Assay
≥98% (HPLC)
form
powder
technique(s)
cell culture | mammalian: suitable
color
yellow
mp
180-181 °C (lit.)
solubility
chloroform: 50 mg/mL
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Furthermore, RA and other retinoids can also inhibit cellular proliferation and stimulate tyrosinase activity in a human melanoma cell line, while also inhibiting cell-substrate adhesion and motility in melanocytes. ATRA plays a vital role in the formation of the mammalian vascular system. Specifically, it regulates endothelial cell proliferation and vascular remodeling throughout tissue angiogenesis.
Application
- Retinoic acid (RA) has been used for the differentiation of embryonic stem cells into motor neurons.
- It has been used for the differentiation of Xenopus ectoderm into pancreas.
- It has also been used to study epigenetic regulation by RARα (retinoic acid receptor α).
- It has been used to study RA signaling of prospermatogonia transition into spermatogonia.
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1B - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Derivation and characterization of functional human neural stem cell derived oligodendrocyte progenitor cells (OPCs) that efficiently myelinate primary neurons in culture.
Protocols
Step-by-step culture protocols for neural stem cell culture including NSC isolation, expansion, differentiation and characterization.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service