A0637
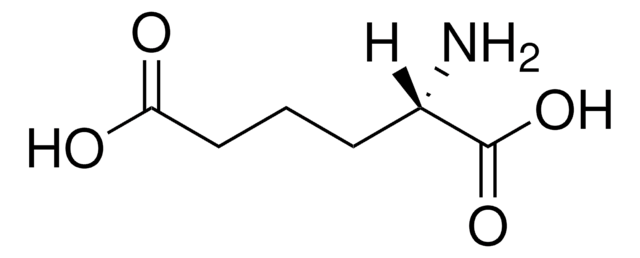
DL-2-Aminoadipic acid
≥99% (TLC), powder, gliotoxic
Synonym(s):
DL-α-Aminoadipic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HO2C(CH2)3CH(NH2)CO2H
CAS Number:
Molecular Weight:
161.16
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Product Name
DL-2-Aminoadipic acid, ≥99%
Quality Level
Assay
≥99%
form
powder
mp
196-198 °C (lit.)
storage temp.
2-8°C
SMILES string
NC(CCCC(O)=O)C(O)=O
InChI
1S/C6H11NO4/c7-4(6(10)11)2-1-3-5(8)9/h4H,1-3,7H2,(H,8,9)(H,10,11)
InChI key
OYIFNHCXNCRBQI-UHFFFAOYSA-N
Gene Information
rat ... Grin2b(24410)
Looking for similar products? Visit Product Comparison Guide
Application
DL-2-Aminoadipic acid (AAA) has been used as an astrotoxin to kill astrocytes that prevent efficient integration of transplanted cells into the retina. It has also been used as a gliotoxin to study the effect of astrocytic swelling on the tortuosity.
Biochem/physiol Actions
DL-2-Aminoadipic acid (AAA) is a six-carbon homolog of glutamate and a gliotoxic compound. It is generally used to considerably reduce the number of astroglia in cerebellar cultures that acts as a model to study the mechanisms of a-aminoadipic acid induced glial toxicity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Bräuner-Osborne et al.
Journal of medicinal chemistry, 39(16), 3188-3194 (1996-08-02)
The homologous series of acidic amino acids, ranging from aspartic acid (1) to 2-aminosuberic acid (5), and the corresponding series of 3-isoxazolol bioisosteres of these amino acids, ranging from (RS)-2-amino-2-(3-hydroxy-5-methylisoxazol-4-yl)acetic acid (AMAA, 6) to (RS)-2-amino-6-(3-hydroxy-5-methylisoxazol-4-yl)hexanoic acid (10), were tested as
The toxic effect of sodium glutamate and DL-$\alpha$-aminoadipic acid on rat retina: Changes in high affinity uptake of putative transmitters
Karlsen RL, et al.
Journal of Neurochemistry, 31(4), 1055-1061 (1978)
H Q Wu et al.
European journal of pharmacology, 281(1), 55-61 (1995-07-25)
L-alpha-Aminoadipic acid is a lysine metabolite with neuroexcitatory properties, and has previously been shown to inhibit the production of the broad spectrum excitatory amino acid receptor antagonist kynurenic acid in brain tissue slices. The effects of L-alpha-aminoadipic acid on the
Huadong Ni et al.
Journal of neuroinflammation, 16(1), 1-1 (2019-01-05)
Despite accumulating evidence on the role of glial cells and their associated chemicals in mechanisms of pain, few studies have addressed the potential role of chemokines in the descending facilitation of chronic pain. We aimed to study the hypothesis that
Gliotoxin-induced swelling of astrocytes hinders diffusion in brain extracellular space via formation of dead-space microdomains
Sherpa AD, et al.
Glia, 62(7), 1053-1065 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service