104655
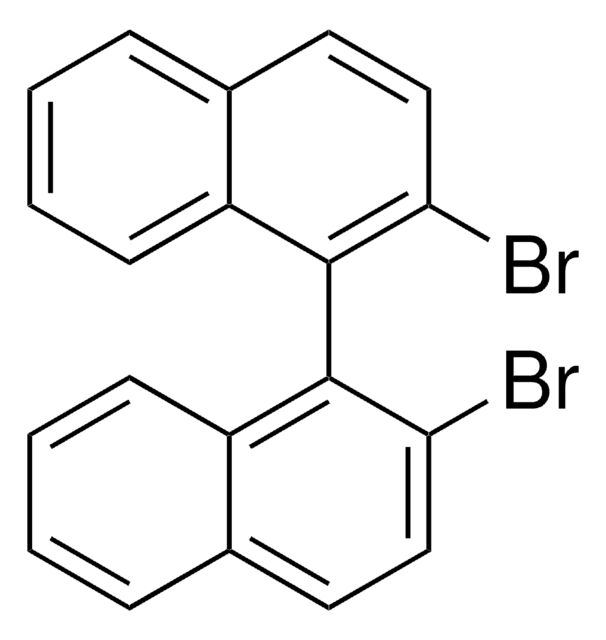
1,1′-Bi-2-naphthol
99%
동의어(들):
(±)-1,1′-Binaphthalene-2,2′-diol, (±)-2,2′-Dihydroxy-1,1′-dinaphthyl, 2,2′-Dihydroxybinaphthalene, 2,2′-Dihydroxydinaphthyl, 2,2′-Dinaphthol, BINOL
로그인조직 및 계약 가격 보기
크기 선택
모든 사진(3)
크기 선택
보기 변경
About This Item
Linear Formula:
HOC10H6C10H6OH
CAS Number:
Molecular Weight:
286.32
Beilstein:
997518
EC Number:
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
반응 적합성
reagent type: ligand
mp
214-217 °C (lit.)
SMILES string
Oc1ccc2ccccc2c1-c3c(O)ccc4ccccc34
InChI
1S/C20H14O2/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22H
InChI key
PPTXVXKCQZKFBN-UHFFFAOYSA-N
애플리케이션
Chiral ligand and auxiliary for asymmetric Michael addition reaction; enantioselective Diels-Alder reaction; alkynylation.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Yifeng Zhou et al.
Organic letters, 6(23), 4147-4149 (2004-11-05)
The readily available and inexpensive (S)-BINOL ligand in combination with Ti(O(i)Pr)(4) is an effective chiral catalyst for the catalytic asymmetric addition of alkynylzinc to unactivated simple ketones. Good to excellent enantioselectivities were achieved. No previous case has been reported successfully
BINOL: a versatile chiral reagent.
Jean Michel Brunel
Chemical reviews, 105(3), 857-897 (2005-03-10)
Shanshan Yu et al.
The Journal of organic chemistry, 76(8), 2814-2819 (2011-03-17)
The fluorescent properties of a series of H(8)BINOL-amine compounds are investigated. It is revealed that the intramolecular hydrogen bonds of these compounds contribute to the shift of the emission of their H(8)BINOL unit to a much longer wavelength. That is
Liheng Feng et al.
Organic & biomolecular chemistry, 9(8), 2938-2942 (2011-03-08)
A glucose sensing switch is formed by water soluble conjugated polymer (PP-S-BINOL) and boronic acid-functionalized benzyl viologen (o-BBV). The two-component system shows a high sensitivity for glucose sensing with a 17-fold increase in the fluorescence intensity in the presence of
Yolanda Pérez-Fuertes et al.
Nature protocols, 3(2), 210-214 (2008-02-16)
A simple three-component chiral derivatization protocol for determining the enantiopurity of chiral primary amines by 1H NMR spectroscopic analysis is described here. The method involves condensation of the amines with 2-formylphenylboronic acid and enantiopure 1,1'-bi-2-naphthol. This approach affords a mixture
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.