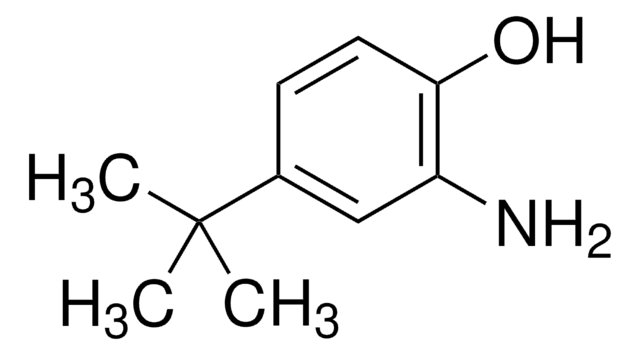
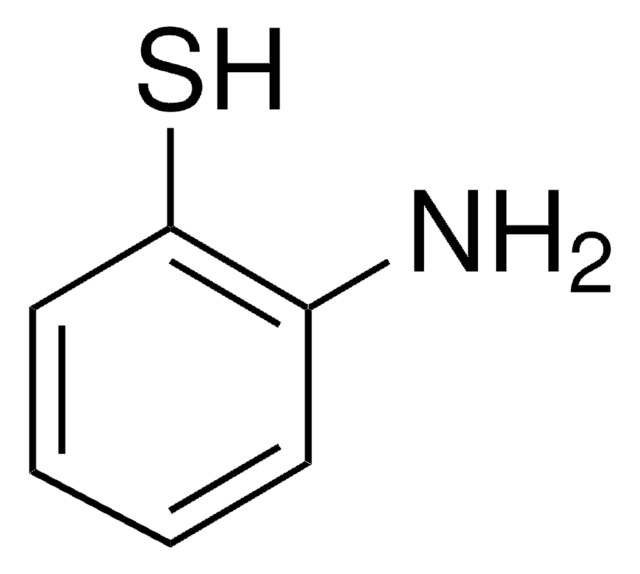
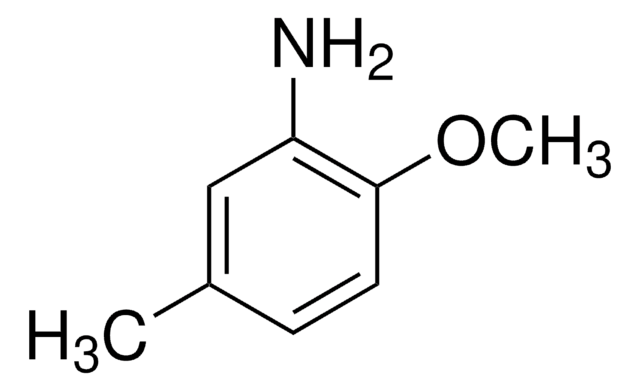
가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
일반 설명
애플리케이션
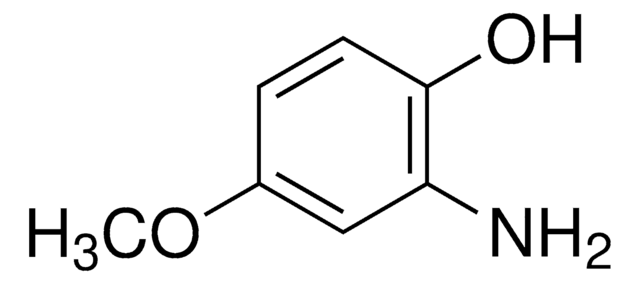
2-Amino-4-methylphenol was used in the synthesis of novel functionalized spiropyran derivatives of 2H-1,3-benzoxazinone series[4].
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Hongmei Peng et al.
Inorganic chemistry, 47(21), 9828-9835 (2008-10-03)
The synthesis and reactivity of a series of sodium and rare-earth metal complexes stabilized by a dianionic N-aryloxo-functionalized beta-ketoiminate ligand were presented. The reaction of acetylacetone with 1 equiv of 2-amino-4-methylphenol in absolute ethanol gave the compound 4-(2-hydroxy-5-methylphenyl)imino-2-pentanone (LH2, 1)
Antony O Bulanov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(3), 1146-1152 (2008-06-14)
Six novel functionalized spiropyran's derivatives of 2H-1,3-benzoxazinone series were synthesized by introducing the substituents with chelating ability into 2H-chromene part of the 8'-formyl-7'-hydroxy-3-methyl-4-oxo-3,4-dihydro-2H-1,3-benzoxazine-2-spiro-2'-[2H]-chromene (I) by condensation with 2-aminophenol, 2-amino-4-methylphenol, 2-amino-4-nitrophenol, 2-amino-1-methylbenzimidazole, 4-amino-4H-1,2,4-triazole, N-(4-aminophenyl)acetamide. (1)H NMR, UV/vis, IR spectroscopy combined with
Z He et al.
Applied and environmental microbiology, 66(7), 3010-3015 (2000-07-06)
In spite of the variety of initial reactions, the aerobic biodegradation of aromatic compounds generally yields dihydroxy intermediates for ring cleavage. Recent investigation of the degradation of nitroaromatic compounds revealed that some nitroaromatic compounds are initially converted to 2-aminophenol rather
A Tomoda et al.
Journal of biochemistry, 110(6), 1004-1007 (1991-12-01)
2-Amino-4-methylphenol was converted to a brownish yellow material by the lysates of human erythrocytes or purified human hemoglobin. The reaction proceeded oxidatively, coupled with the oxidation of hemoglobin. The major component of the brownish yellow material produced by oxidative condensation
M Akazawa et al.
The Tohoku journal of experimental medicine, 192(4), 301-312 (2001-04-05)
When human erythrocytes were incubated with o-aminophenol at pH 7.0 at 37 degrees C for 46 hours, intracellular oxyhemoglobin was completely oxidized to methemoglobin during the initial 6 hours, and methemoglobin formed was then reduced to oxyhemoglobin during the following
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.