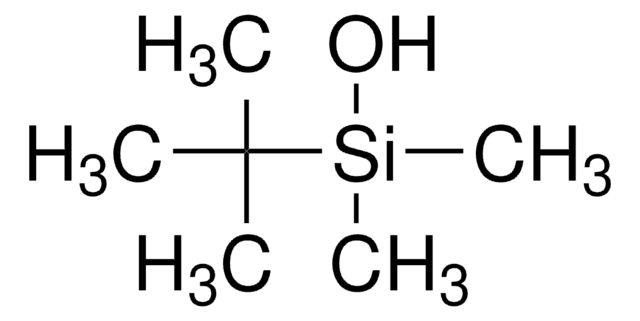190500
tert-Butyldimethylsilyl chloride
reagent grade, 97%
동의어(들):
tert-Butyl(chloro)dimethylsilane, tert-Butyldimethylchlorosilane, TBDMSCl
로그인조직 및 계약 가격 보기
모든 사진(6)
About This Item
Linear Formula:
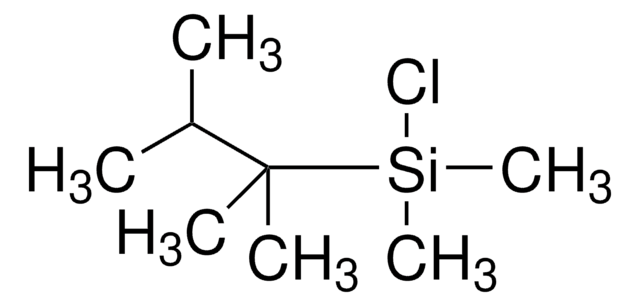
(CH3)3CSi(CH3)2Cl
CAS Number:
Molecular Weight:
150.72
Beilstein:
505999
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
reagent grade
Quality Level
분석
97%
양식
solid
bp
125 °C (lit.)
mp
86-89 °C (lit.)
SMILES string
CC(C)(C)[Si](C)(C)Cl
InChI
1S/C6H15ClSi/c1-6(2,3)8(4,5)7/h1-5H3
InChI key
BCNZYOJHNLTNEZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
tert-Butyldimethylsilyl chloride (TBDMSCl) is an organosilicon compound that can be used as a versatile protecting reagent for alcohols, amines, amides, and various carboxylic acids. It is also used in the preparation of isoxazoline N-oxides from α-bromonitroalkanes and olefins. TBDMSCl is used as a precursor that forms a silicon substrate for Ag/ZnO/graphene-based nanocomposites to form effective photocatalytic system for hydrogen production.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Eye Dam. 1 - Flam. Sol. 1 - Skin Corr. 1A
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 2
Flash Point (°F)
71.6 °F - closed cup
Flash Point (°C)
22 °C - closed cup
이미 열람한 고객
Journal of the Chemical Society. Chemical Communications, 125-125 (1993)
Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups, 1999
Makino T and Toyota S
Bulletin of the Chemical Society of Japan, 78(5), 917-928 (2005)
Effective silylation of carboxylic acids under solvent-free conditions with tert-butyldimethylsilyl chloride (TBDMSCL) and triisopropylsilyl chloride (TIPSCL)
Firouzabadi H, et al.
Phosphorus, Sulfur, and Silicon and the Related Elements, 166(1) (2000)
Ag/ZnO/graphene-tert-butyldimethylsilyl chloride hybrid nanocomposite as highly efficient catalyst for hydrogen production
Attia YA
Materials Express, 6(3) (2016)
Roman A Kunetsky et al.
Organic letters, 5(25), 4907-4909 (2003-12-05)
A strategy for the synthesis of isoxazoline-N-oxides (cyclic five-membered nitronates) from primary nitro compounds and olefins is described. The key step of the process involves 1,3-dipolar cycloaddition of corresponding 1-bromosilyl nitronates with alkenes. [reaction: see text]
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.