246948
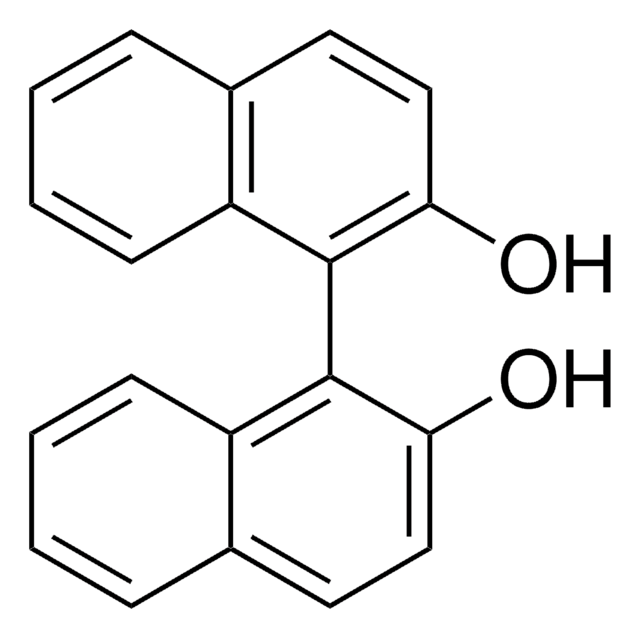
(R)-(+)-1,1′-Bi(2-naphthol)
99%
동의어(들):
(+)-2,2′-Dihydroxy-1,1′-dinaphthyl, (R)-(+)-1,1′-Binaphthalene-2,2′-diol, (R)-BINOL
About This Item
추천 제품
Quality Level
분석
99%
양식
solid
광학 활성
[α]21/D +34°, c = 1 in THF
광학 순도
ee: 99% (HPLC)
mp
208-210 °C (lit.)
SMILES string
Oc1ccc2ccccc2c1-c3c(O)ccc4ccccc34
InChI
1S/C20H14O2/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22H
InChI key
PPTXVXKCQZKFBN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
문서
We present an article concerning BINOL and Derivatives.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.