47695
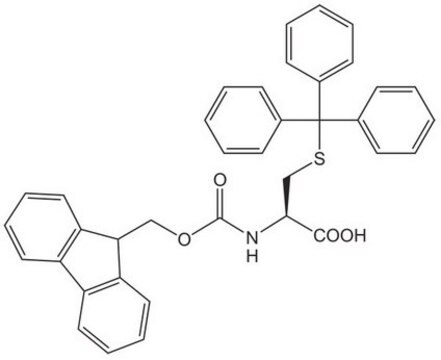
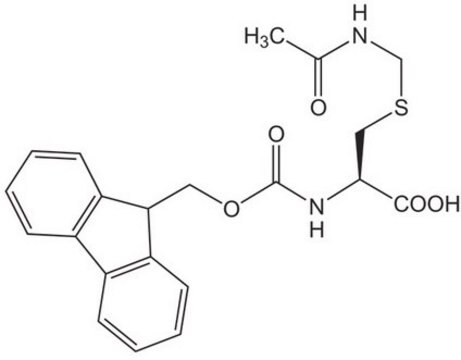
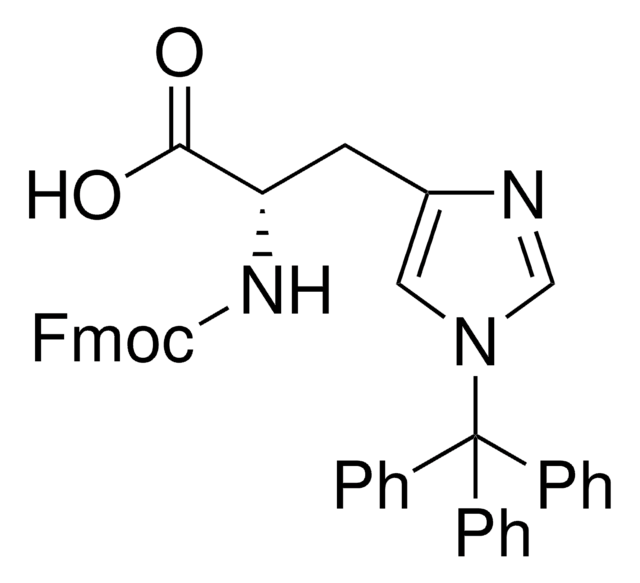
Fmoc-Cys(Trt)-OH
≥95.0% (sum of enantiomers, HPLC)
동의어(들):
N-(9-Fluorenylmethoxycarbonyl)-S-trityl-L-cysteine, Nα-Fmoc-S-trityl-L-cysteine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C37H31NO4S
CAS Number:
Molecular Weight:
585.71
Beilstein:
4221286
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
추천 제품
Quality Level
분석
≥95.0% (sum of enantiomers, HPLC)
광학 활성
[α]20/D +16.0±2°, c = 1% in THF
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
mp
170-173 °C (lit.)
응용 분야
peptide synthesis
작용기
Fmoc
저장 온도
2-8°C
SMILES string
OC(=O)[C@H](CSC(c1ccccc1)(c2ccccc2)c3ccccc3)NC(=O)OCC4c5ccccc5-c6ccccc46
InChI
1S/C37H31NO4S/c39-35(40)34(38-36(41)42-24-33-31-22-12-10-20-29(31)30-21-11-13-23-32(30)33)25-43-37(26-14-4-1-5-15-26,27-16-6-2-7-17-27)28-18-8-3-9-19-28/h1-23,33-34H,24-25H2,(H,38,41)(H,39,40)/t34-/m0/s1
InChI key
KLBPUVPNPAJWHZ-UMSFTDKQSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Fmoc-Cys(Trt)-OH is an amino acid commonly used in the Fmoc solid-phase peptide synthesis.
애플리케이션
Fmoc-Cys(Trt)-OH is an N-terminal protected cysteine derivative used in peptide synthesis. Some of the examples are:
- Synthesis of mono- and bi-functionalized platinum(IV) complexes to target angiogenic tumor vasculature.
- Synthesis of proteins through native chemical ligation of peptide hydrazides as thioester surrogates via solid-phase synthesis.
- Synthesis of glycoconjugates by conjugating reducing sugars to cysteine residues of peptides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Conjugated platinum (IV)? peptide complexes for targeting angiogenic tumor vasculature.
Mukhopadhyay S, et al.
Bioconjugate Chemistry, 19(1), 39-49 (2007)
Parallel synthesis of structurally diverse aminobenzimidazole tethered sultams and benzothiazepinones
Sureshbabu D et al.
Tetrahedron Letters, 53, 6897-6900 (2012)
Panchada Ch V Govindu et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 18(1), 198-207 (2018-11-14)
Avobenzone is the most widely used UVA filter in sunscreen lotion and it is prone to degradation in the presence of sunlight/UV radiation. To overcome the photo-instability of avobenzone, various photostabilizers have been used as additives, including antioxidants such as
Chemical synthesis of proteins using peptide hydrazides as thioester surrogates.
Zheng J S, et al.
Nature Protocols, 8(12), 2483-2483 (2013)
A double-click approach to the protecting group free synthesis of glycoconjugates.
Alexander S R, et al.
Organic & Biomolecular Chemistry, 16(8), 1258-1262 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.